Scientific gadgets for curious minds
The world's first series of pocket-sized radiation detectors and spectrometers, engineered for all natural science enthusiasts
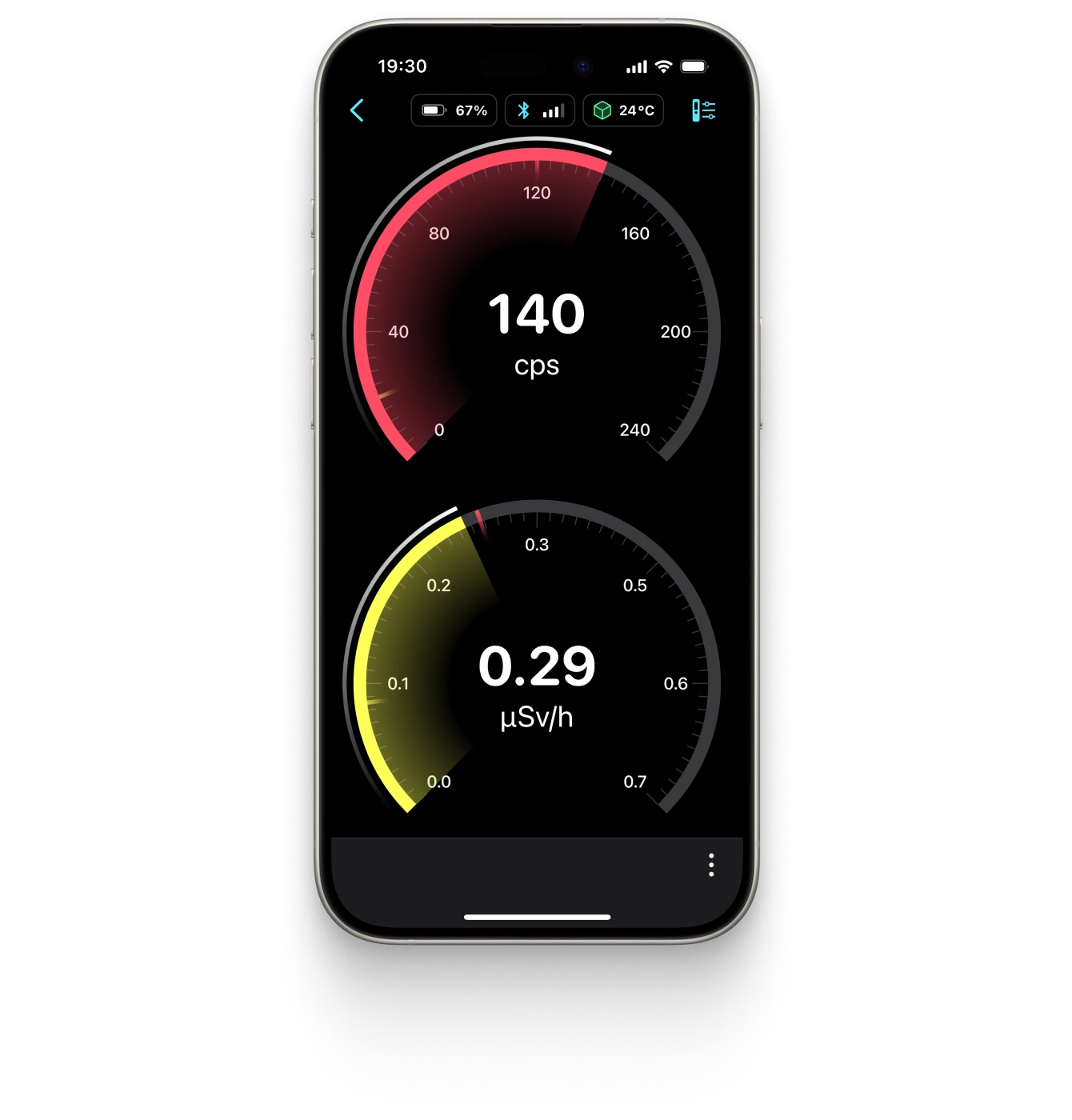
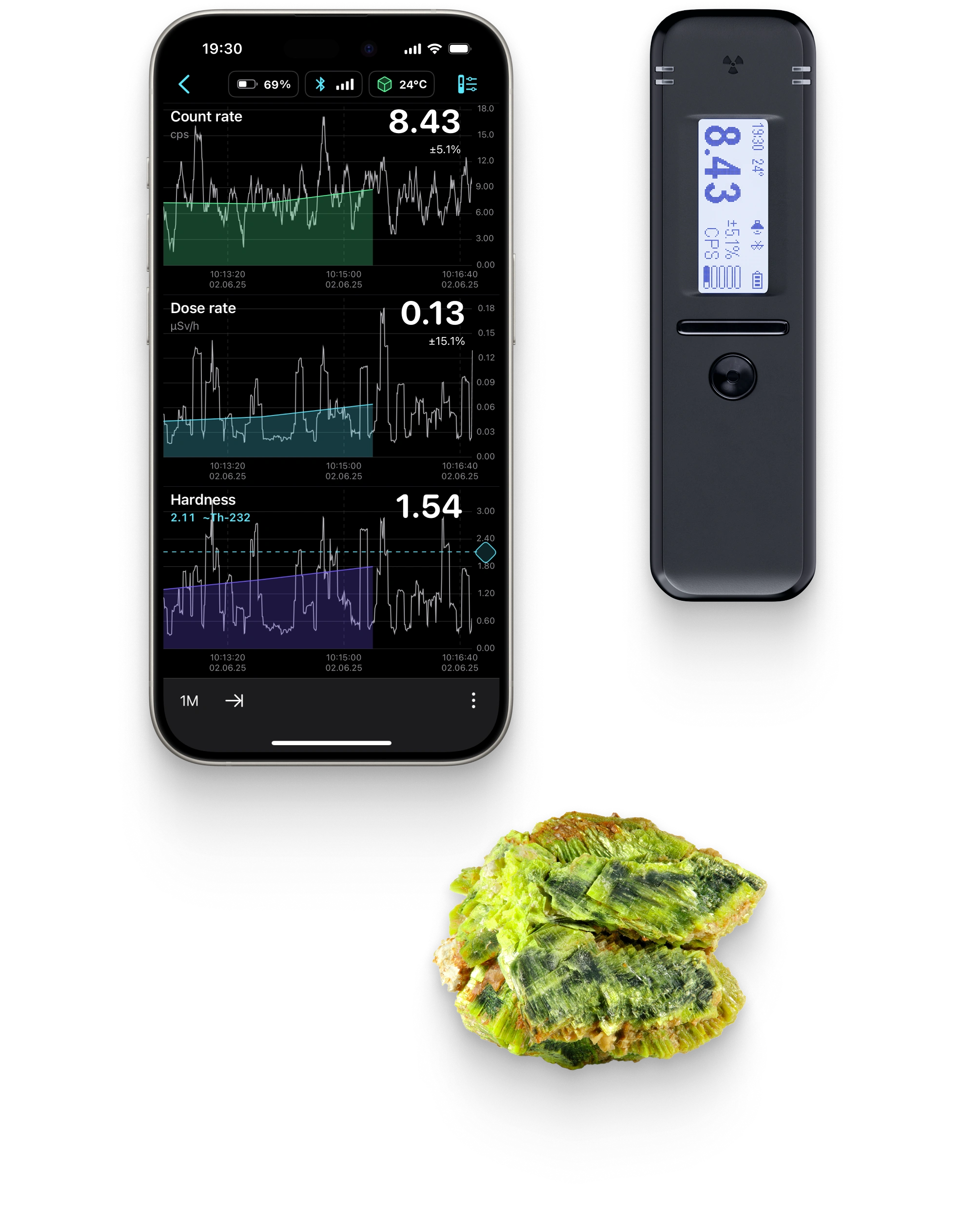
Ultrafast sensitive scintillation detector
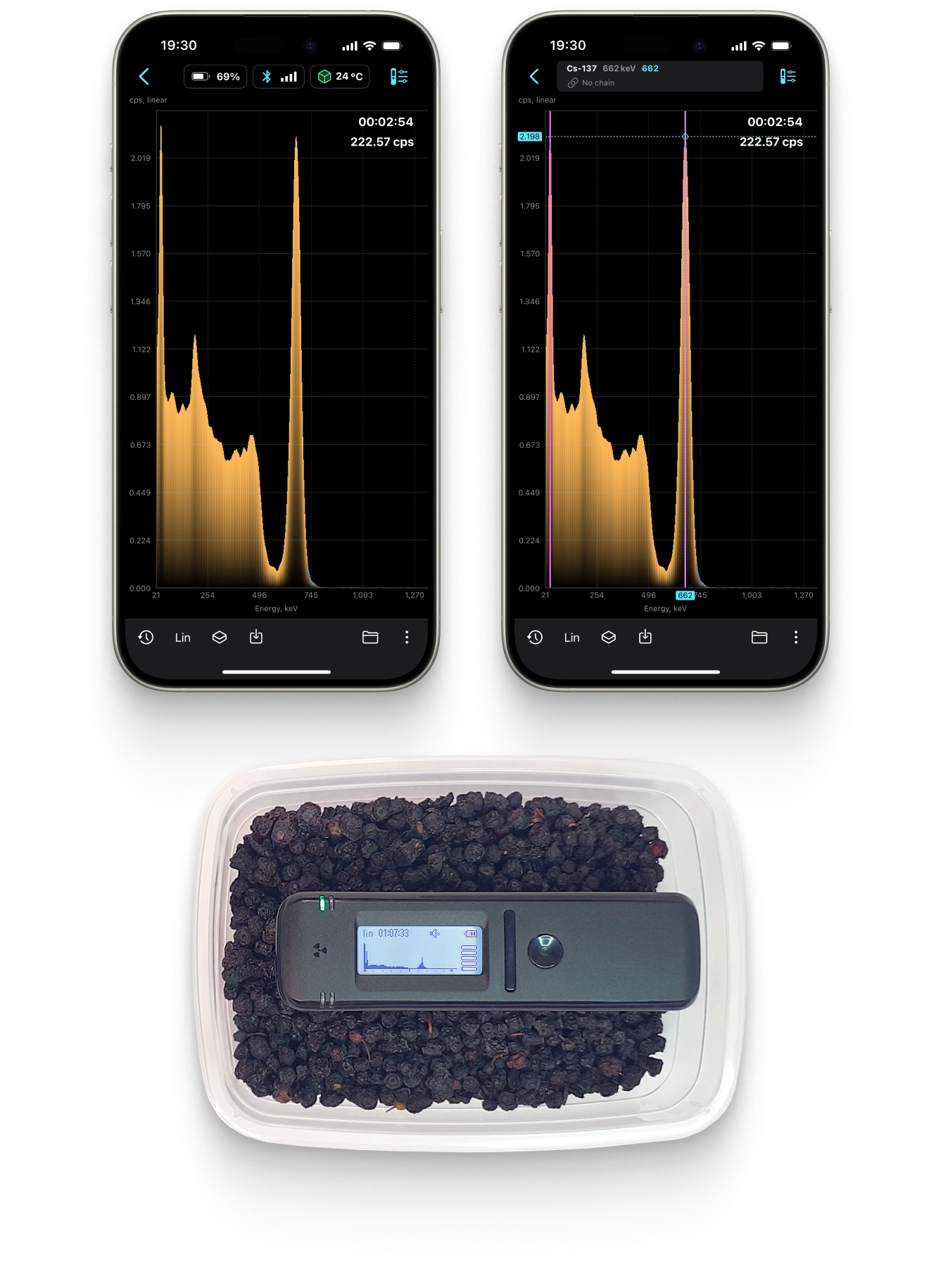
Isotope Identifier and spectrum analyser
FWHM from 7.4% ±0.3%
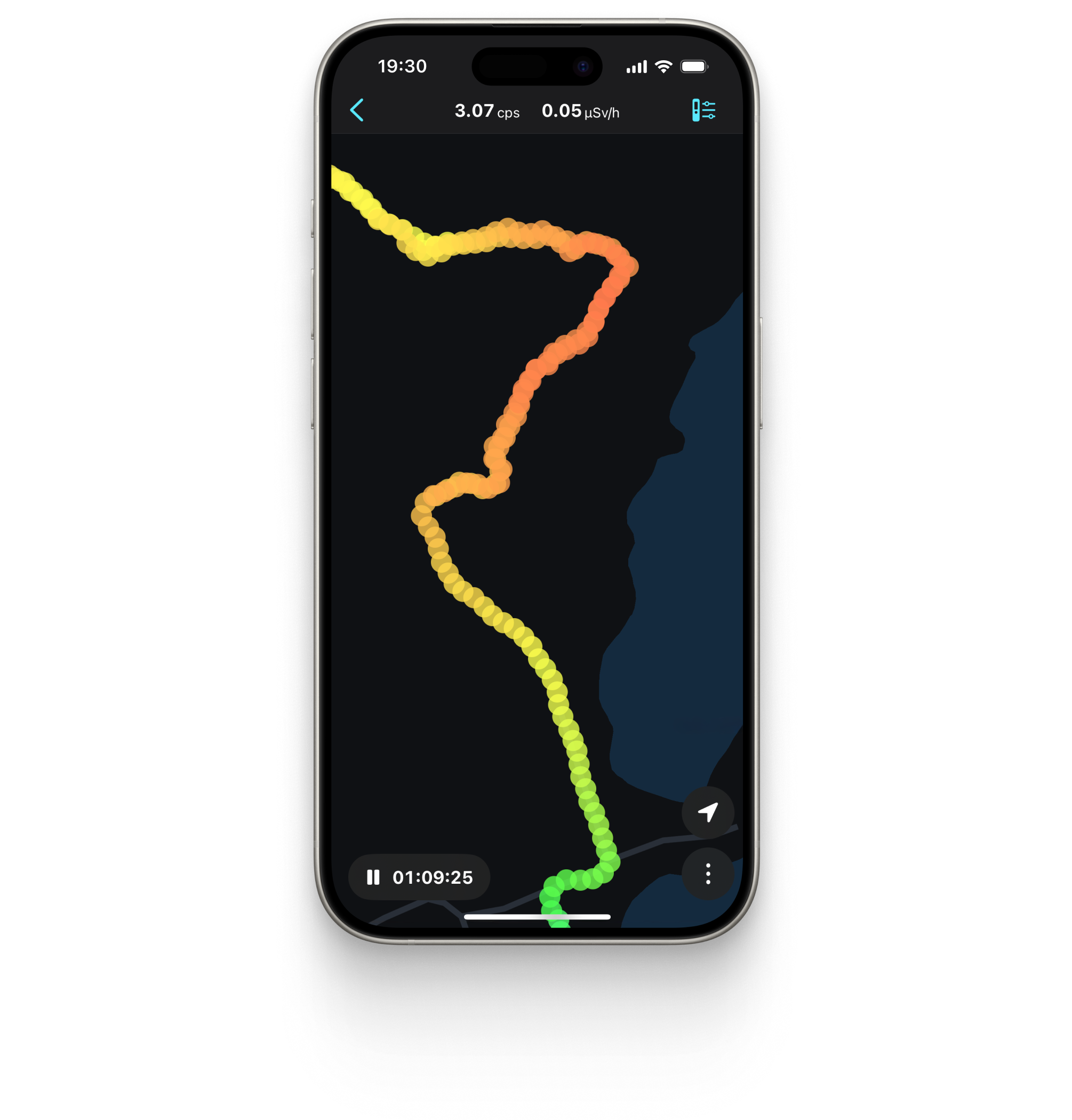
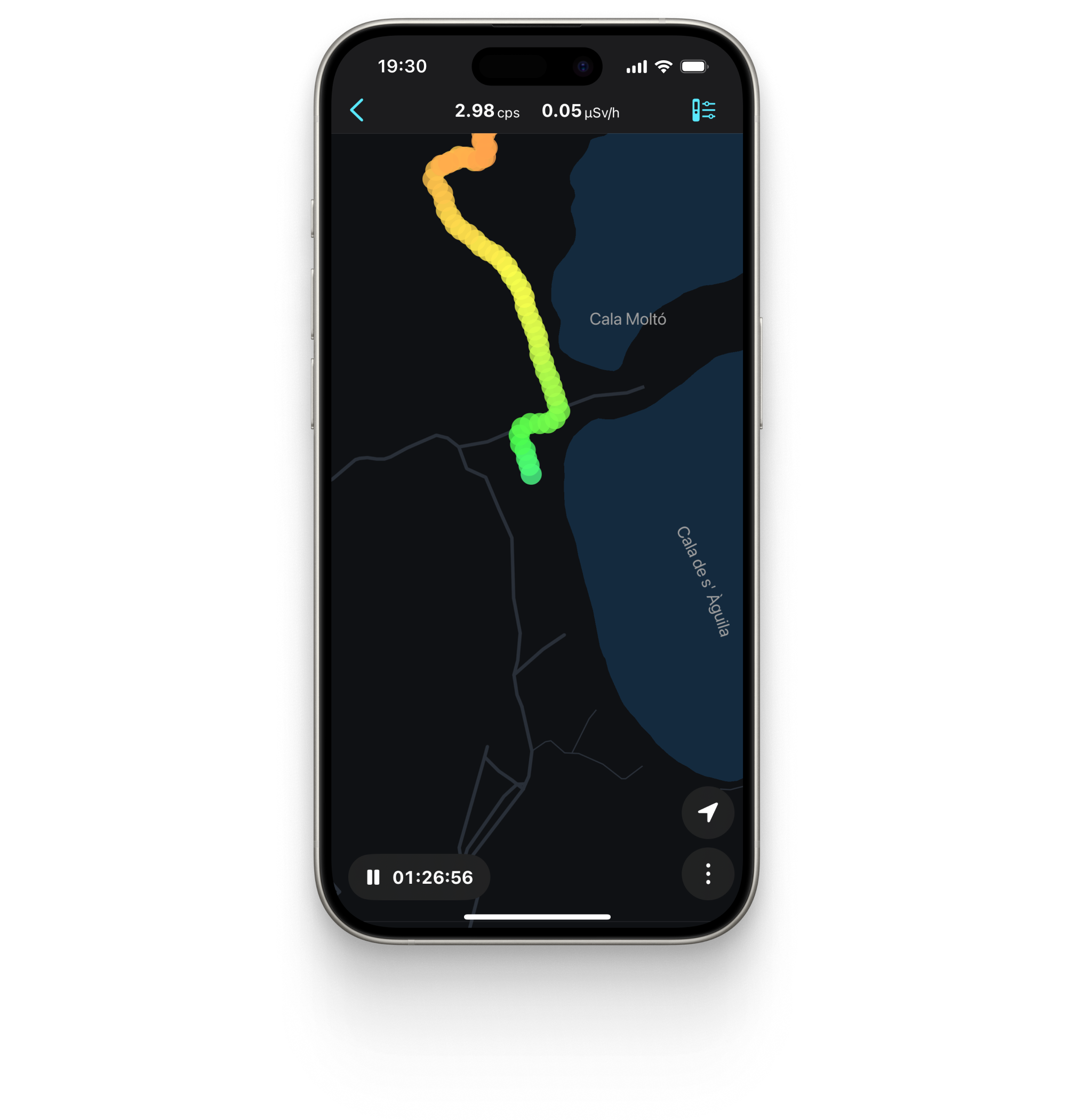
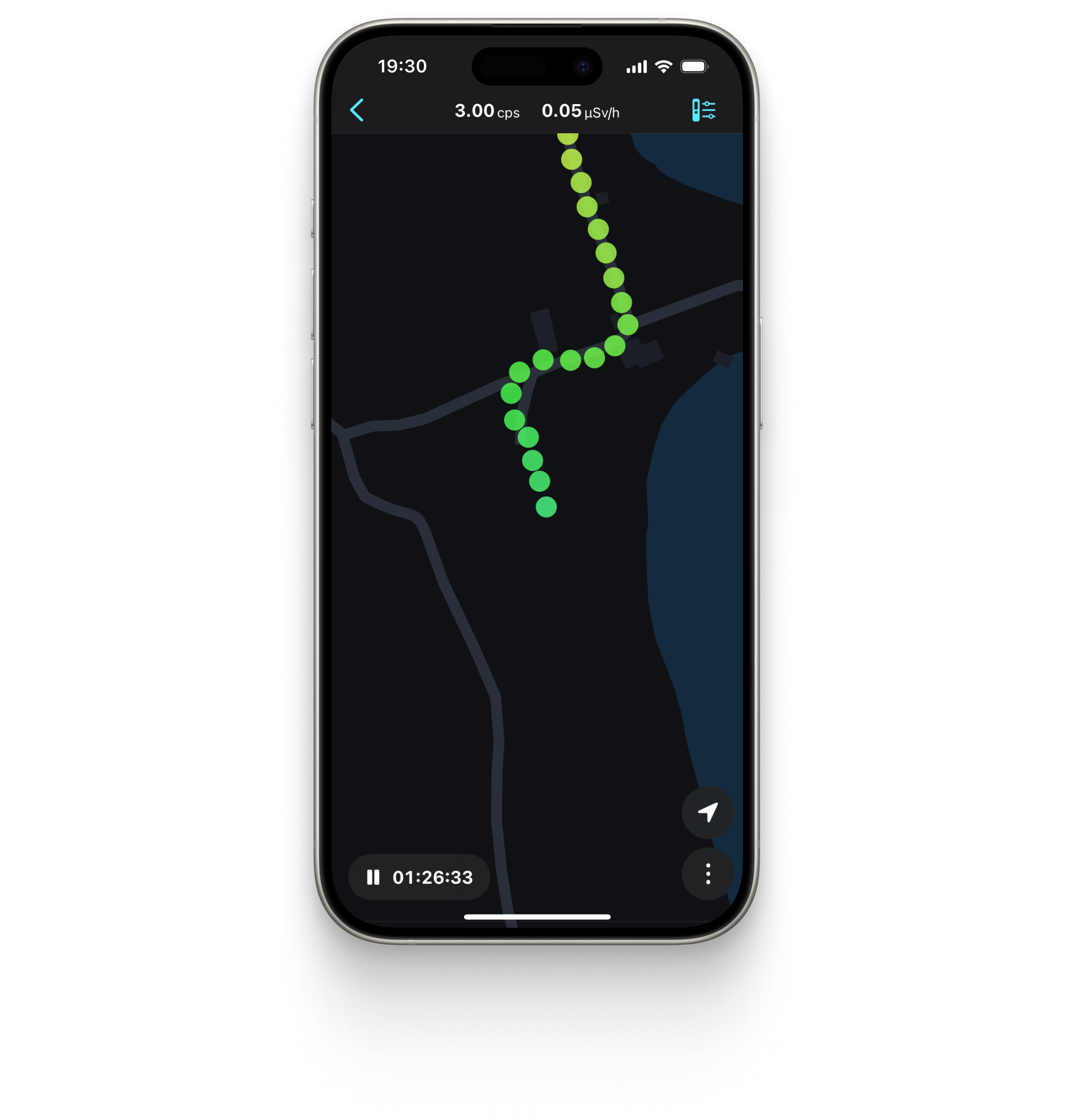
Radiation tracks with Google Maps
Energy and temperature compensated dose rate and spectrum
Food testing mode for contamination
Mobile and PC application with extra features
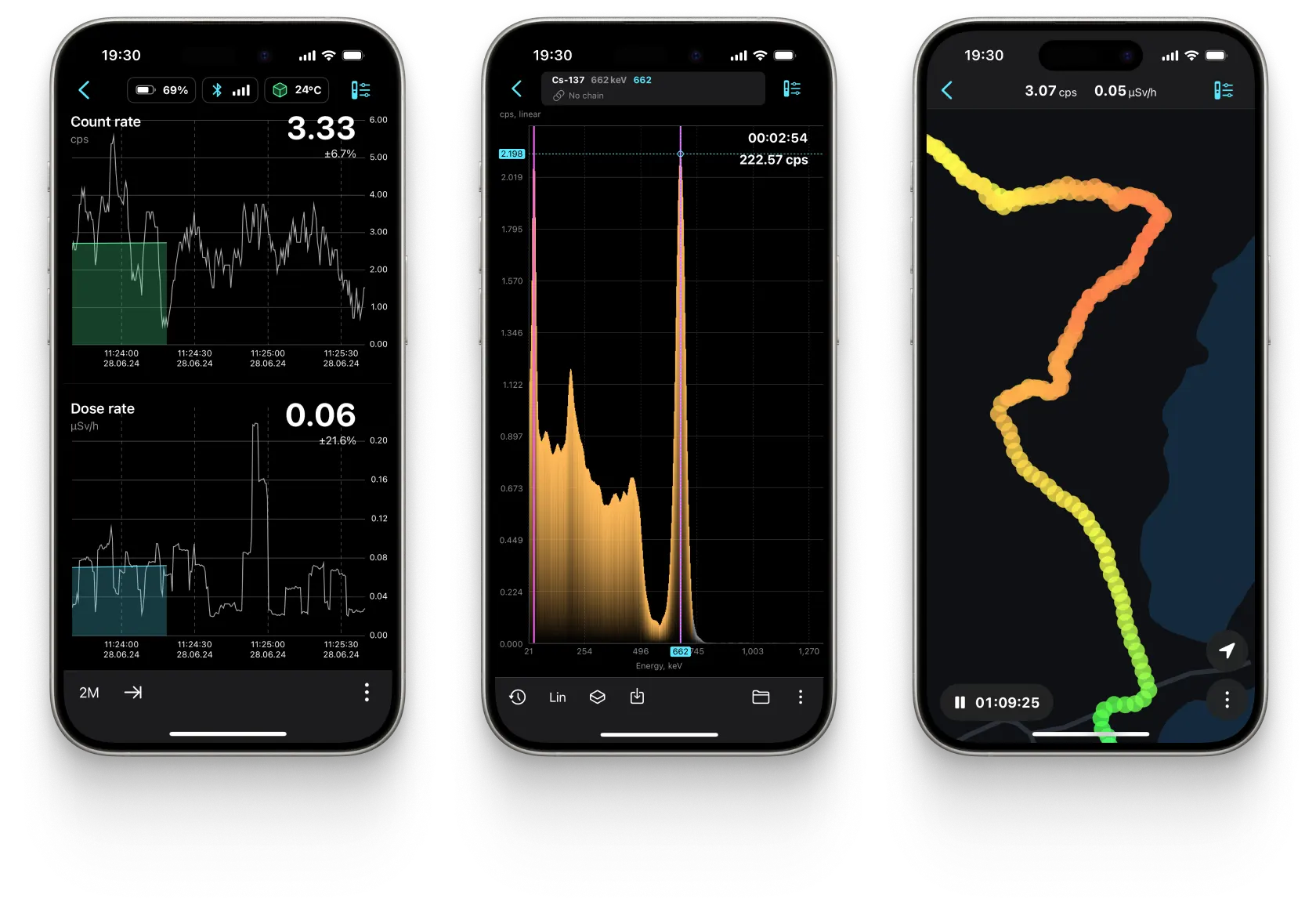
Dashboard
Radiation Level
Dose
Tracking
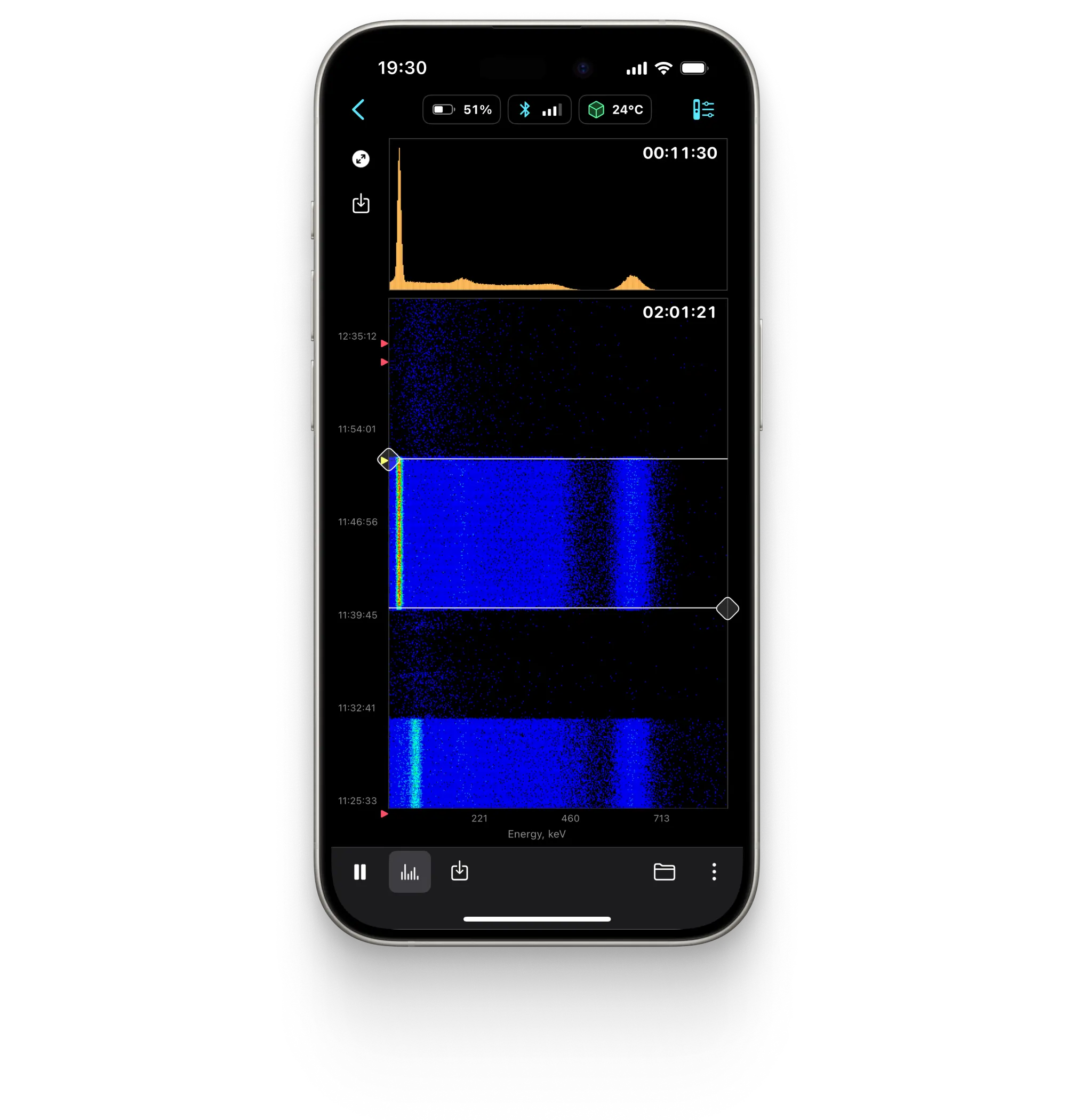
Spectrum
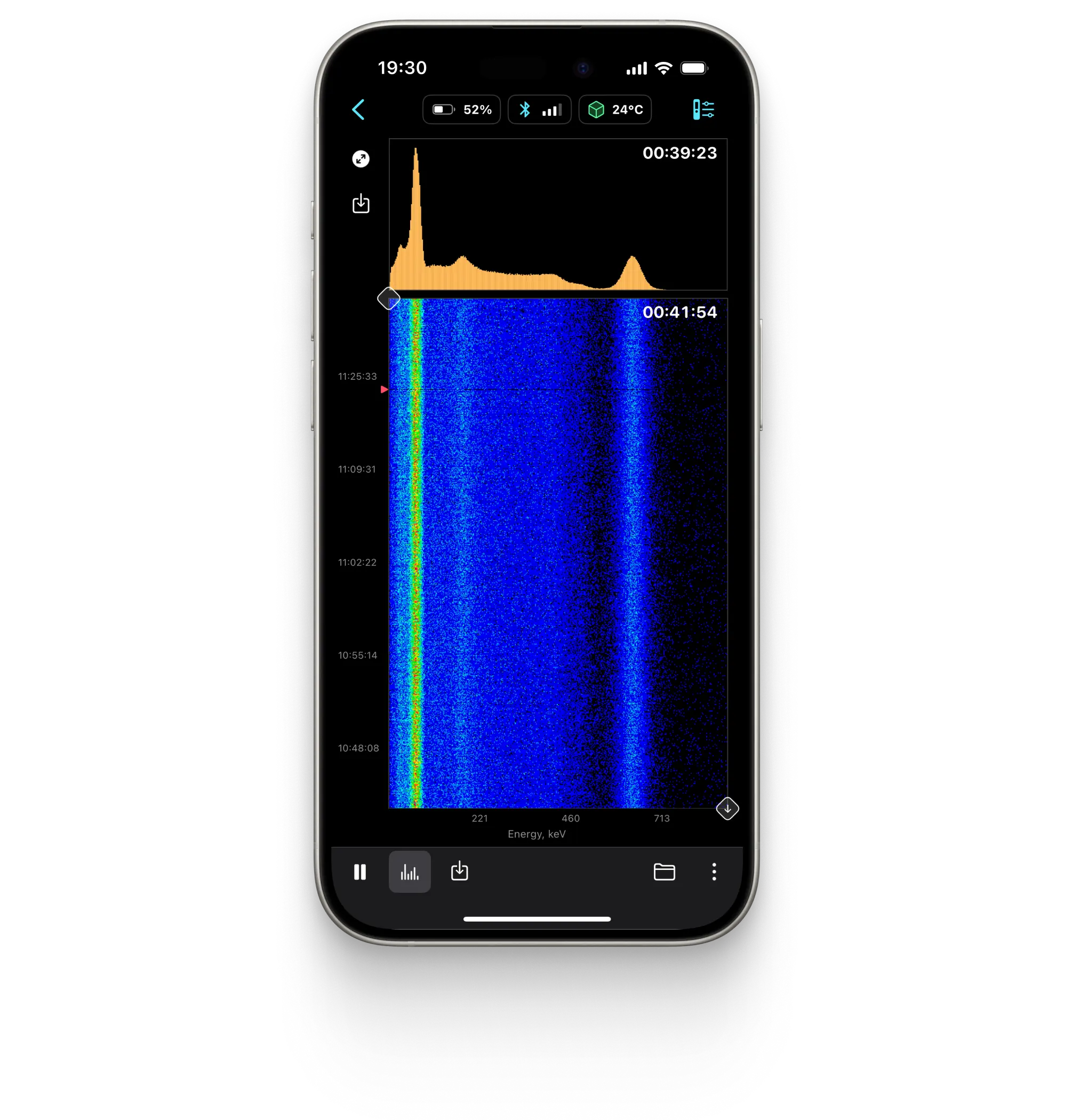
Spectrogram
Event log
Search Mode

Ultrafast sensitive scintillation detector

Isotope Identifier and spectrum analyser

FWHM from 7.4% ±0.3%

Radiation tracks with Google Maps

Energy and temperature compensated dose rate and spectrum

Food testing mode for contamination
Why Radiacode?
Standard Geiger Counter
Results are provided with a delay
Measurements only, no extra features
Space-consuming and hard to carry
Isotopes cannot be identified
Go beyond with Radiacode device
Fast reaction in background radiation
Mobile and PC application with extra features
Portable and versatile
Isotope identification and food analysis
Features

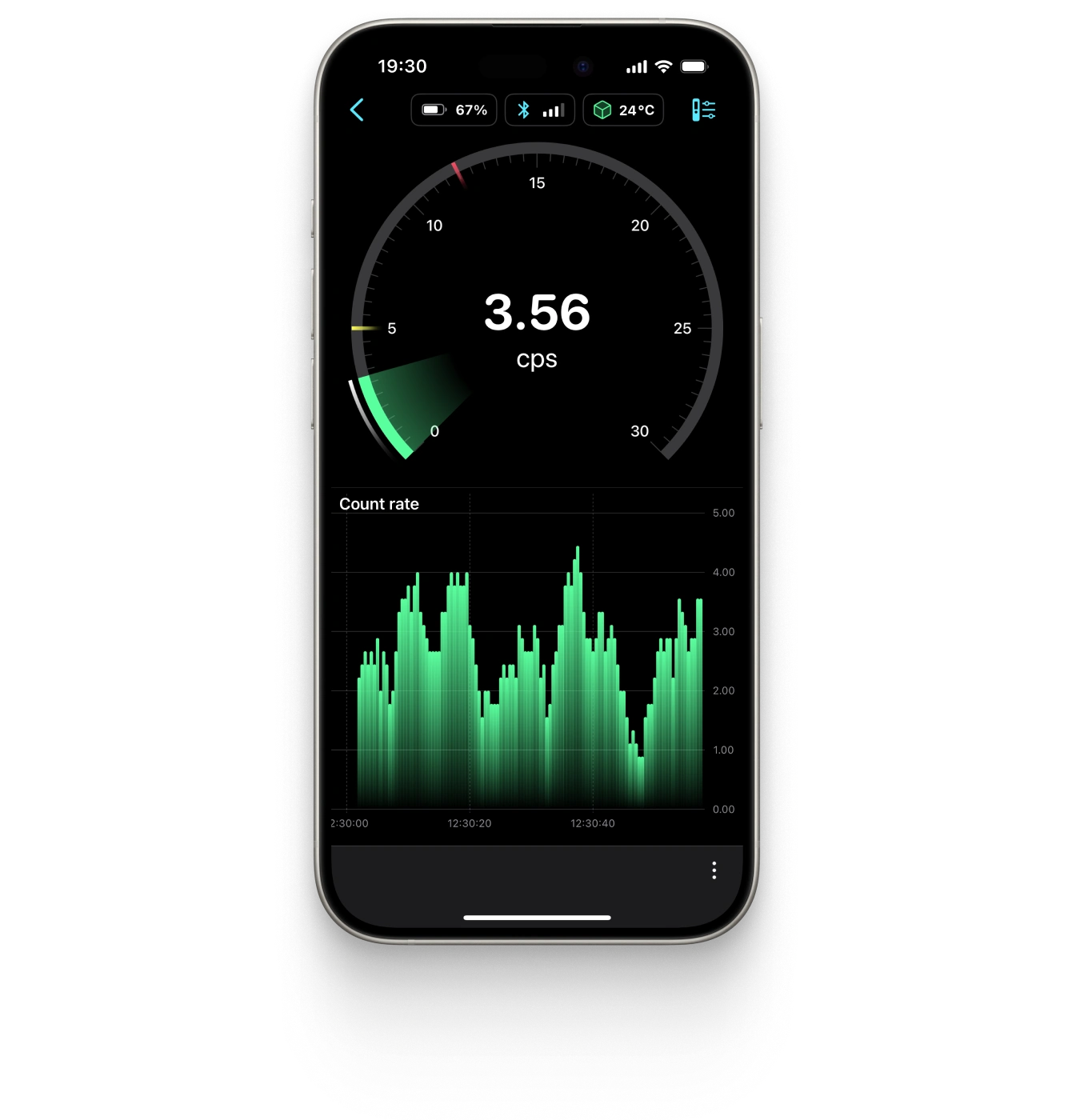
- With real-time updates, readings refresh twice per second, allowing you to cover larger areas in less time without compromising accuracy.
- Enhanced audio feedback, available through your smartphone or headphones, keeps you informed in noisy or sensitive environments without drawing unwanted attention.
- The analog indicator provides peripheral awareness, enabling you to monitor radiation levels without constantly checking the screen, making it easier to react in dynamic situations.
- For those tracking radiation levels over time, the visual graph helps identify rising trends and allows for timely adjustments.
- Additionally, the modified algorithm minimizes response delays, offering faster feedback for split-second decision-making.
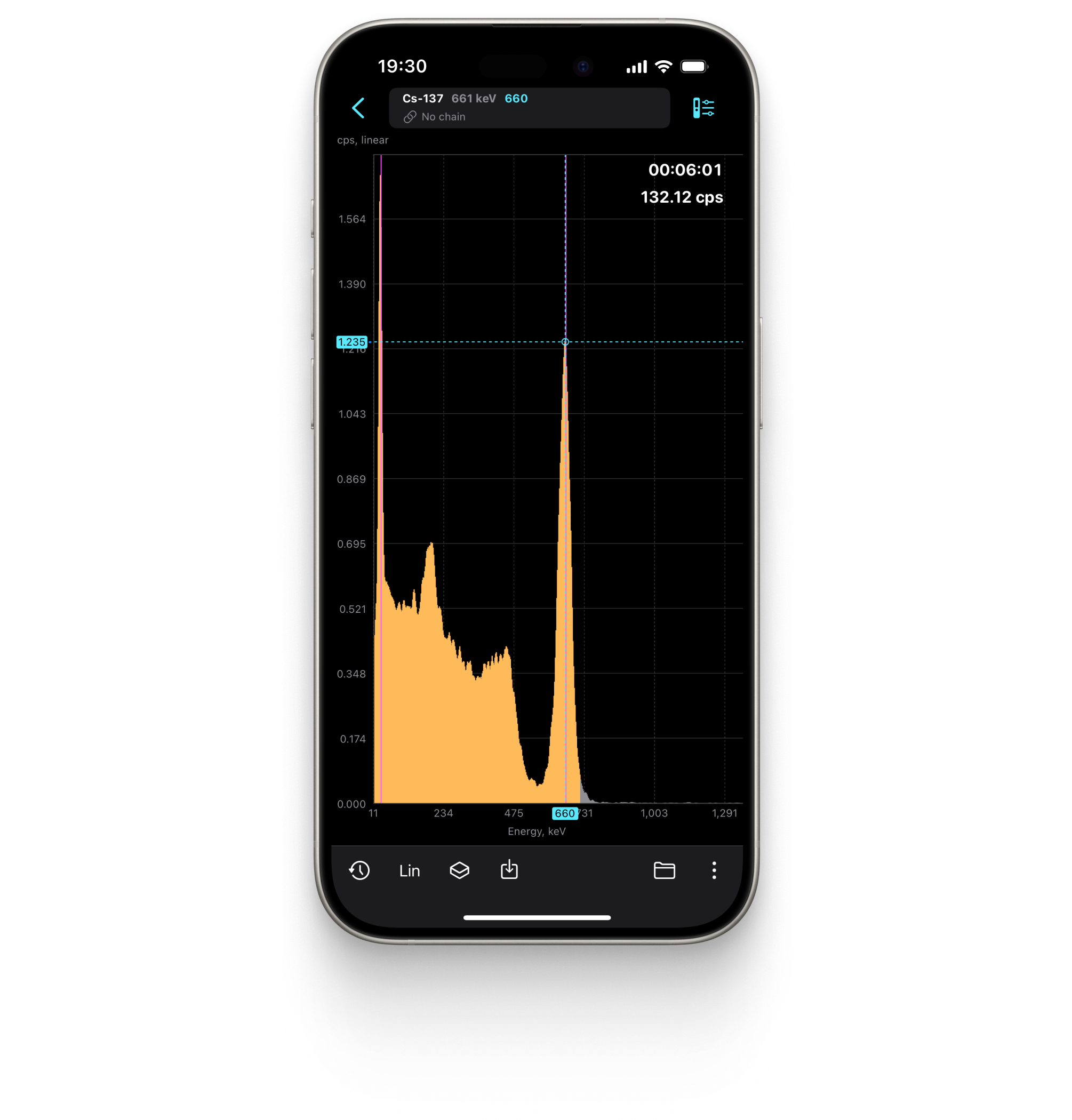
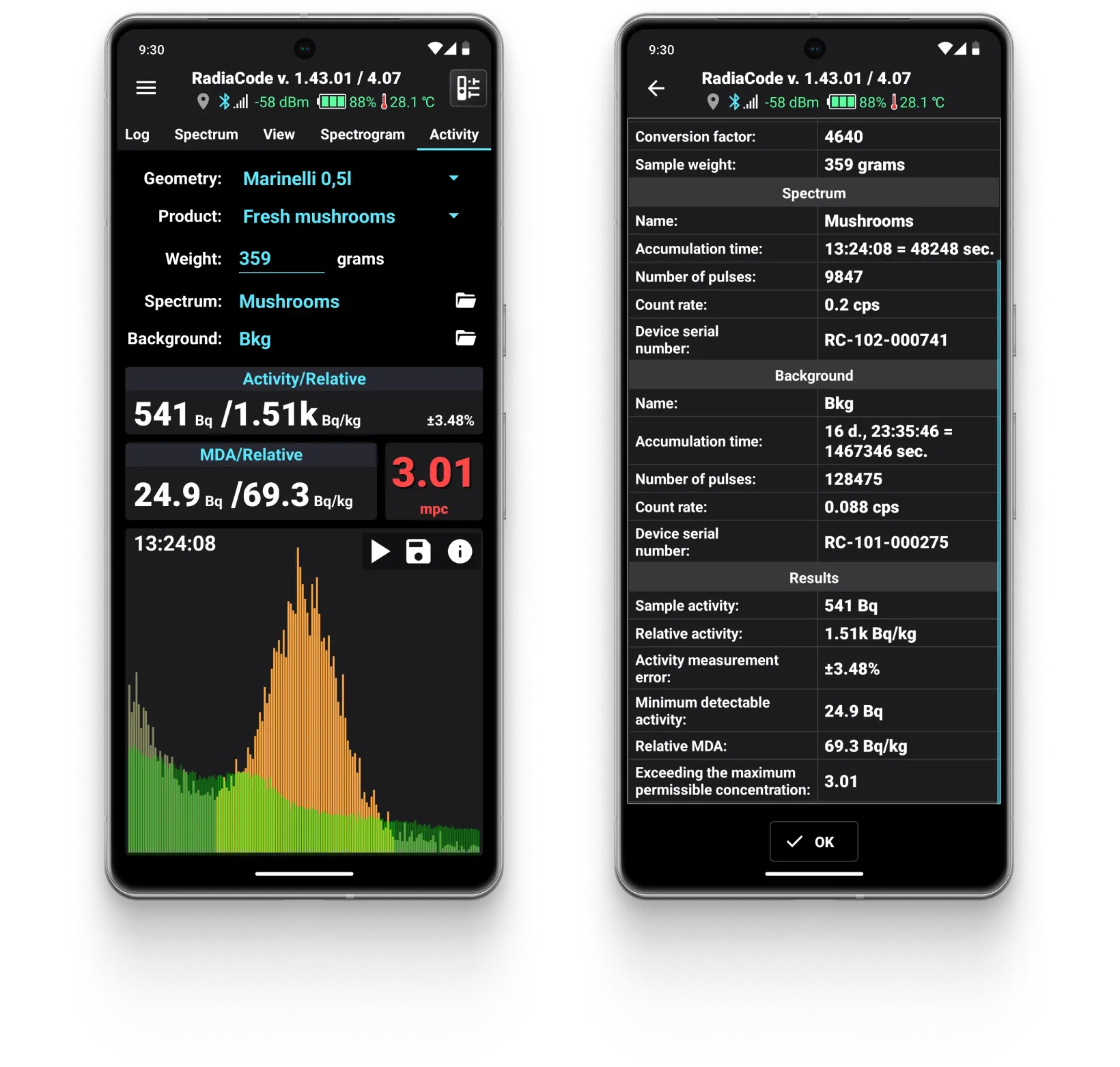
Food Activity Monitoring
Radiacode has a specialized mode for measuring the presence of Cesium-137 in food products.
Chernobyl Rain
Fallout Spots

Mushrooms
Sample activity: 2.34 Bq

Blueberry
Sample activity: 1.77 Bq

Honey
Sample activity: 4.21 Bq

Meat
Sample activity: 3.56 Bq

Mushrooms
Sample activity: 2.34 Bq

Blueberry
Sample activity: 1.77 Bq

Honey
Sample activity: 4.21 Bq

Meat
Sample activity: 3.56 Bq


Worldwide Shipping

2-Year Warranty

Easy Return & Refund
Software
Radiacode devices are engineered for both autonomous operation and enhanced functionality when paired with a smartphone or computer
Discover the full potential of Radiacode, a device designed for smart, flexible, and efficient operations, which transforms into an even more powerful tool when paired with Radiacode software, thereby enhancing device capabilities in radiation monitoring and data analysis.
Hardware
Detector
Display
Case
Battery
CPU & Motherboard
Radiacode Sensor

Moisture-proof sealed capsule containing a CsI(Tl) or GAGG(Ce) crystal.

A precision-calibrated, temperature-compensated power supply assigned to the photomultiplier.

The entire sensor is securely enclosed within a hermetically sealed capsule, precluding any contact of the crystal with the ambient environment.

A high-speed analog-digital circuit, designed to process pulses emerging from the photomultiplier.


Moisture-proof sealed capsule containing a CsI(Tl) or GAGG(Ce) crystal.

A precision-calibrated, temperature-compensated power supply assigned to the photomultiplier.

The entire sensor is securely enclosed within a hermetically sealed capsule, precluding any contact of the crystal with the ambient environment.

A high-speed analog-digital circuit, designed to process pulses emerging from the photomultiplier.
Tech specs
Modes of operations

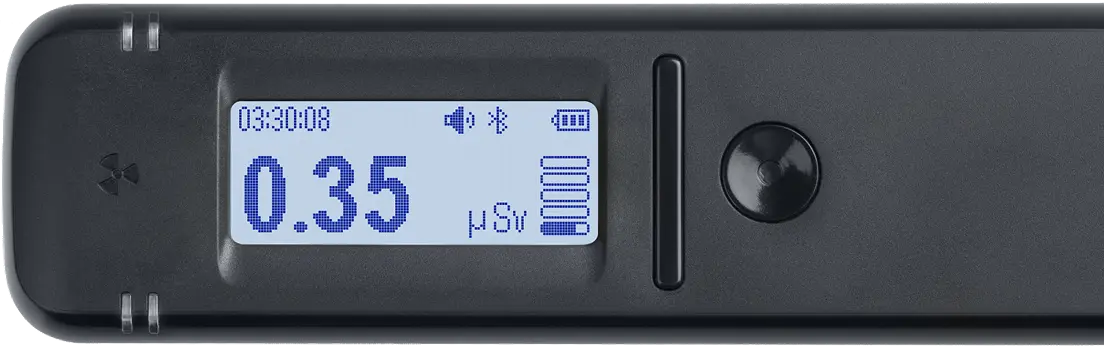
Monitor
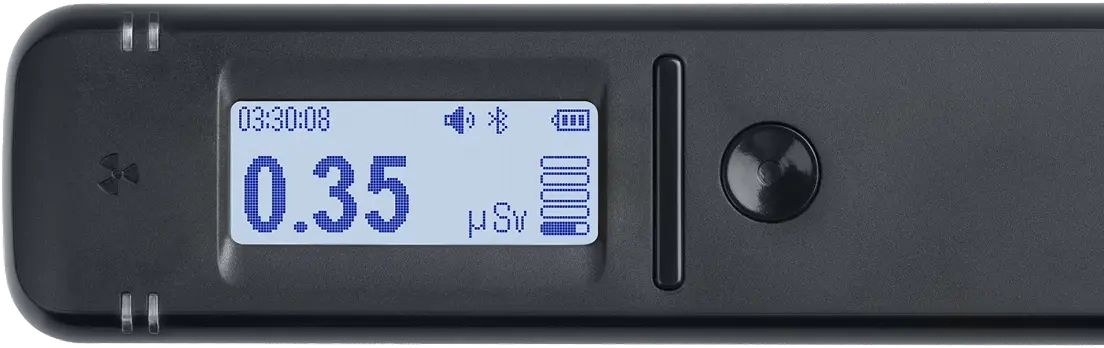
Provides real-time dose rate and count rate measurements (μSv/h, μR/h, CPS, CPM).
Dose
Shows the cumulative dose measurements (μSv, μR).

Search
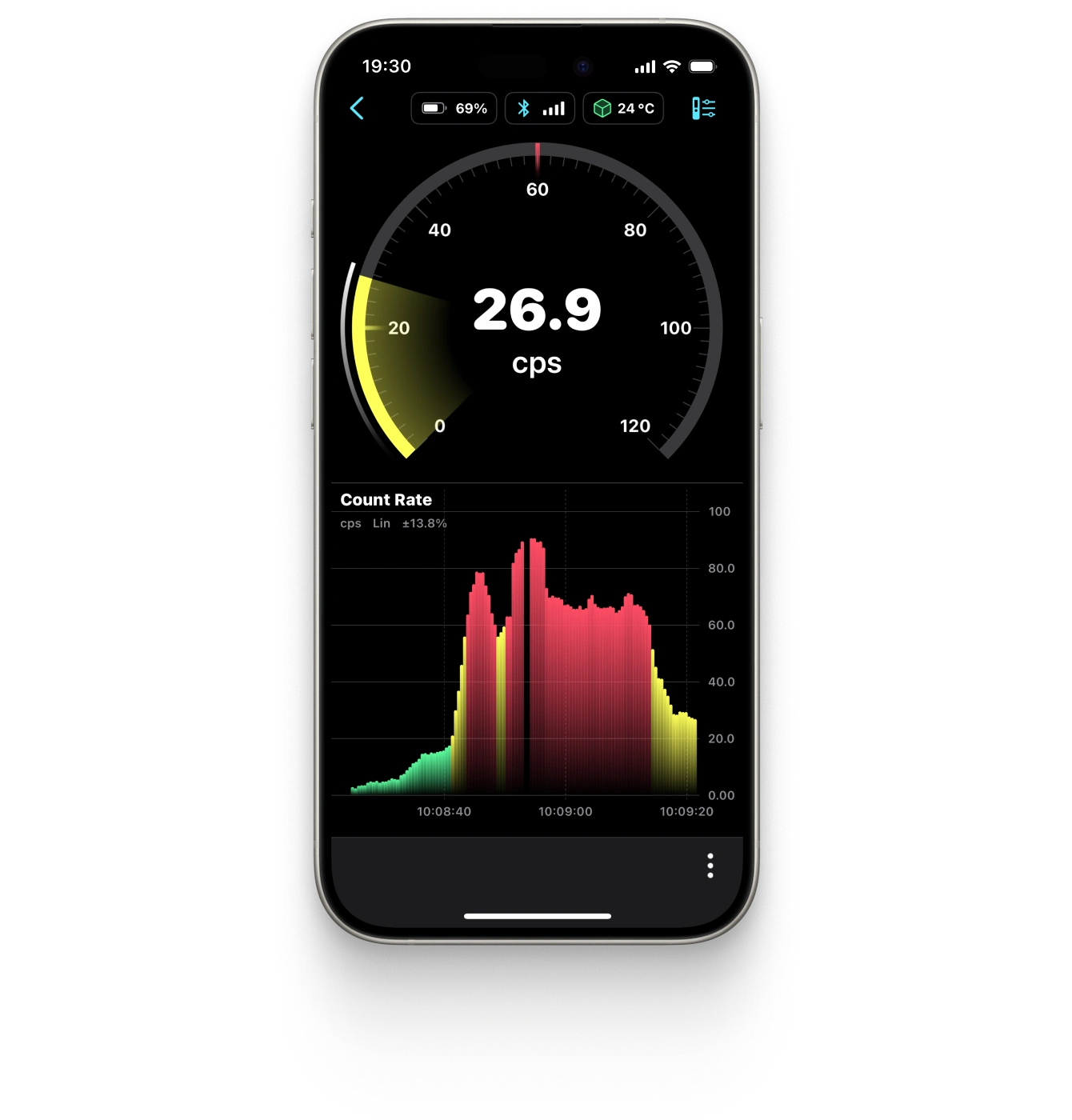
Offers a graphical representation of the count rate (CPM or CPS) aiding in locating point sources of radiation.
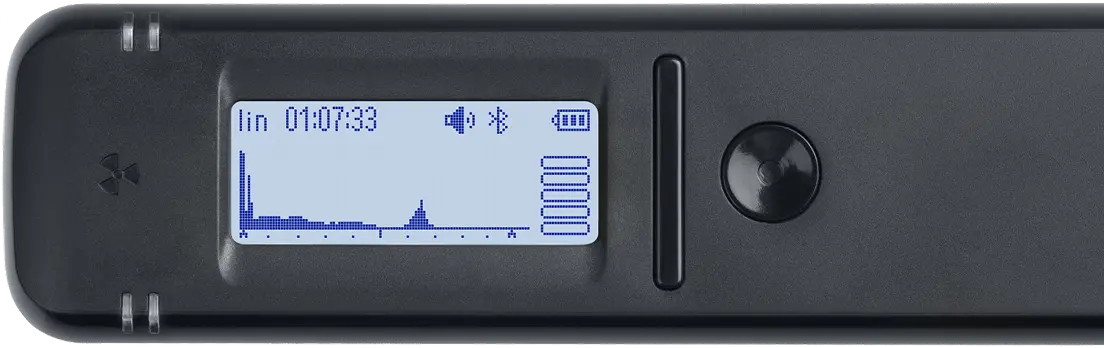
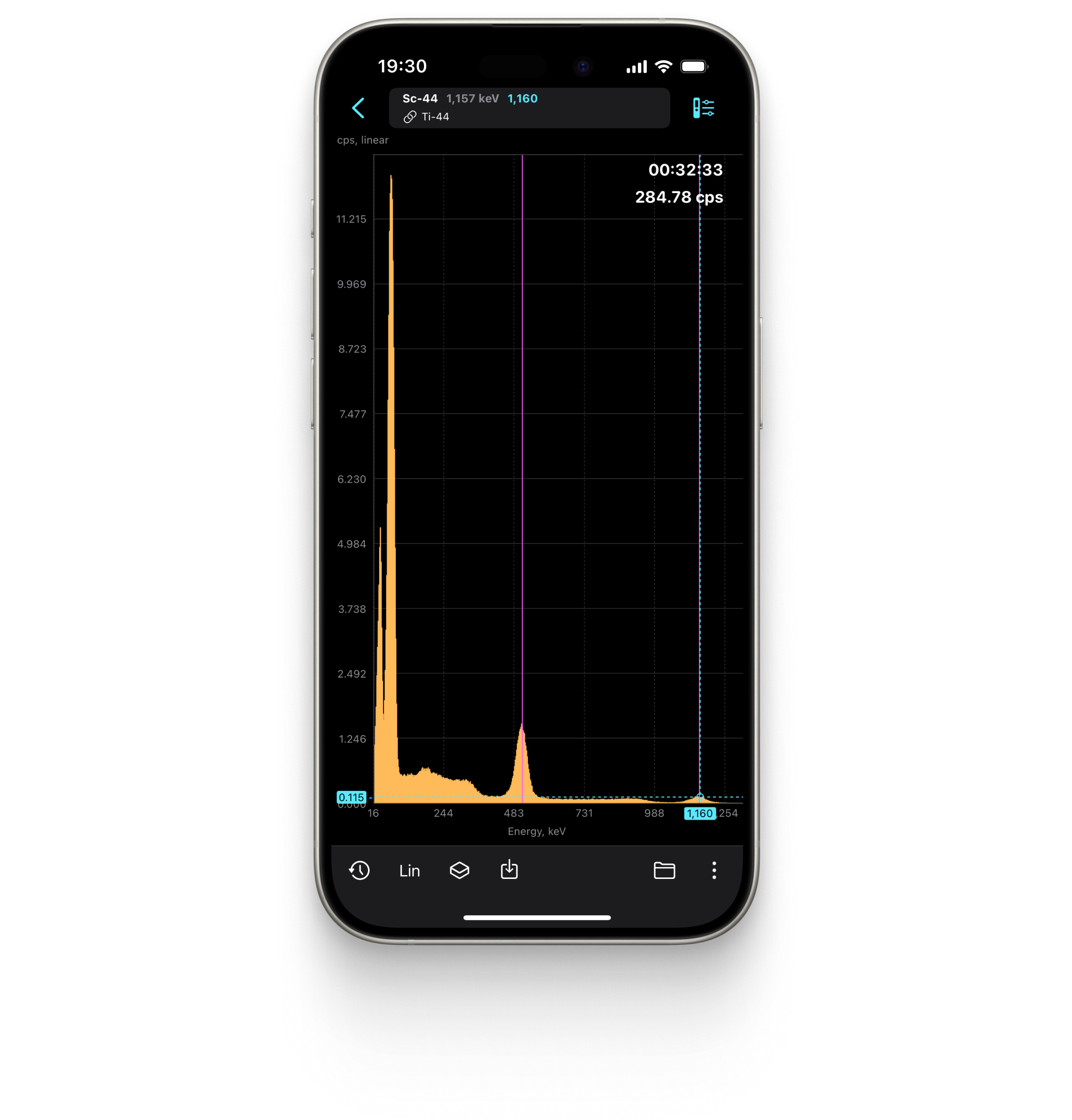
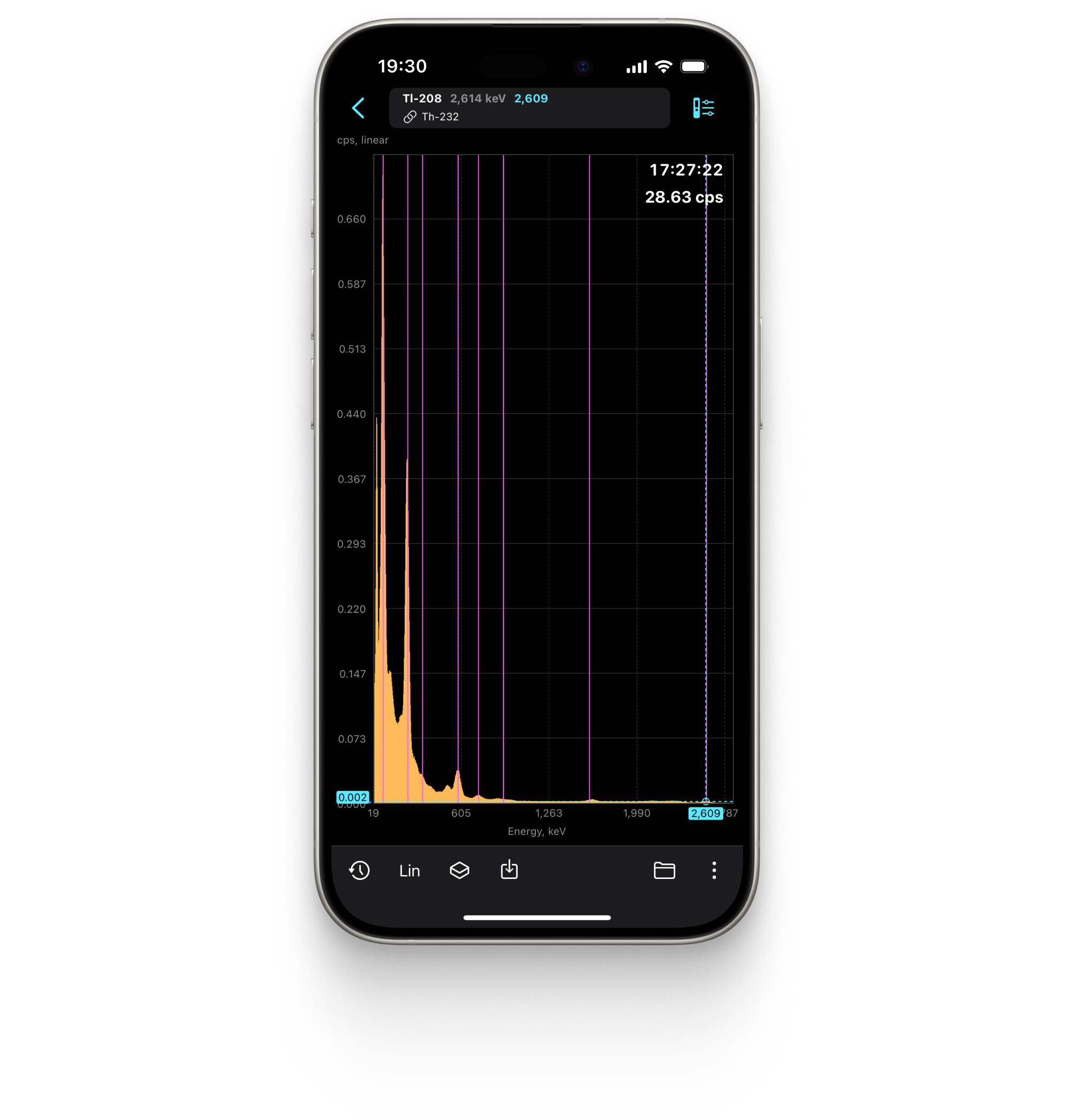
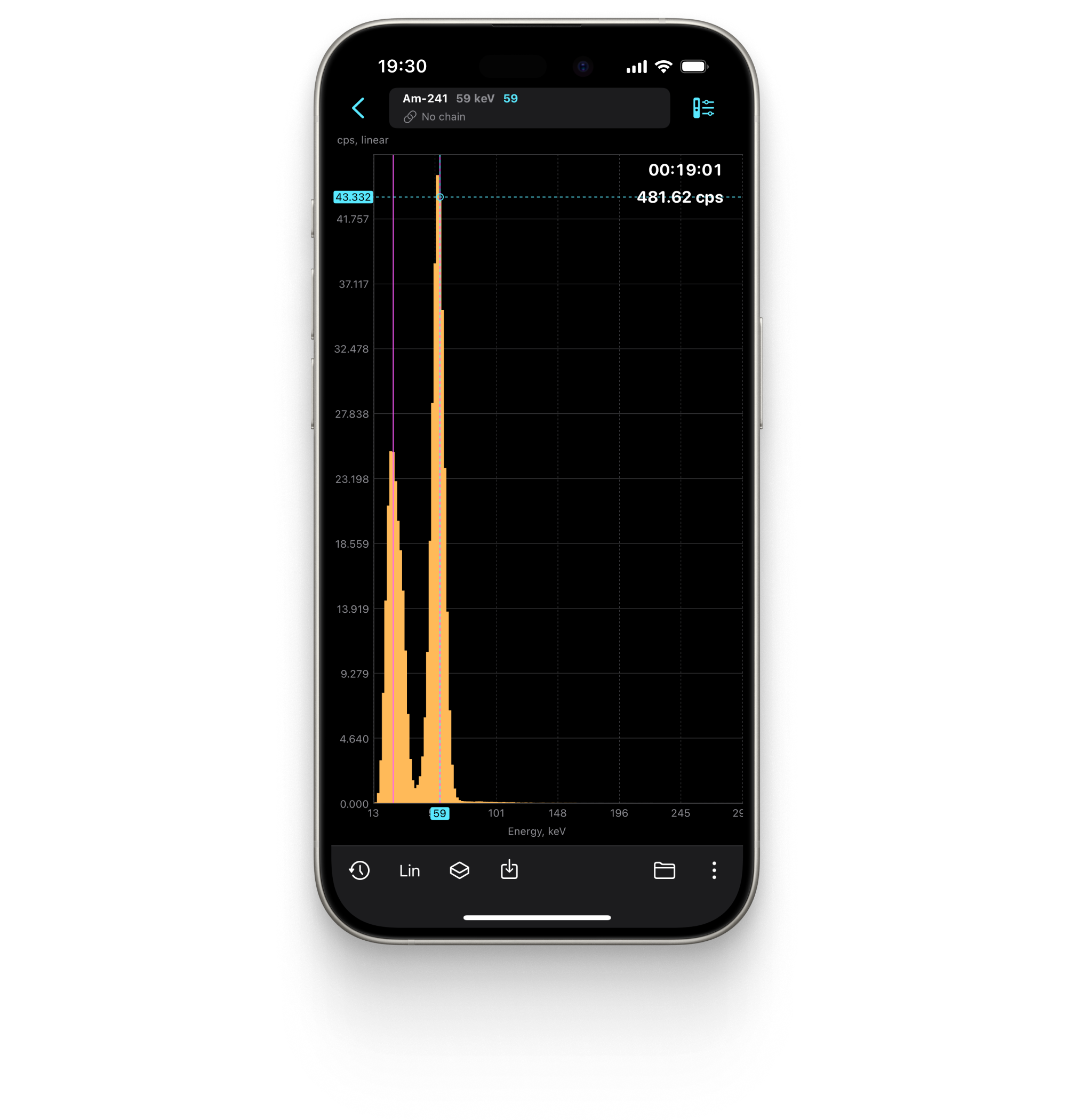
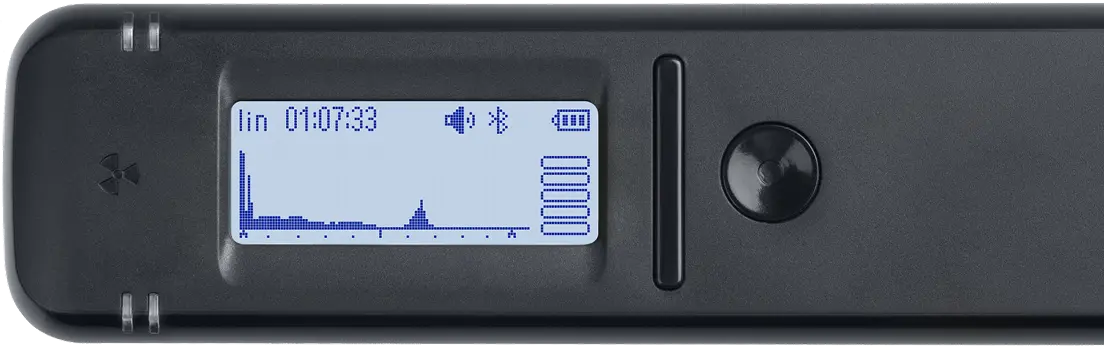
Spectrum
Displays the energy spectrum of gamma radiation on a linear or logarithmic scale.
Your order is in safe hands
Join Community
In synergy with





 Csl (Tl)
Csl (Tl)