
Uranium (without radium impurities) is found in any uranium glass, Red Fiesta glaze, or reagents with uranium compounds.
Over time, radium will begin to accumulate, but it would take millions of years for the spectrum to take on the appearance of natural uranium.

Uranium (without radium impurities) is found in any uranium glass, Red Fiesta glaze, or reagents with uranium compounds.
Over time, radium will begin to accumulate, but it would take millions of years for the spectrum to take on the appearance of natural uranium.
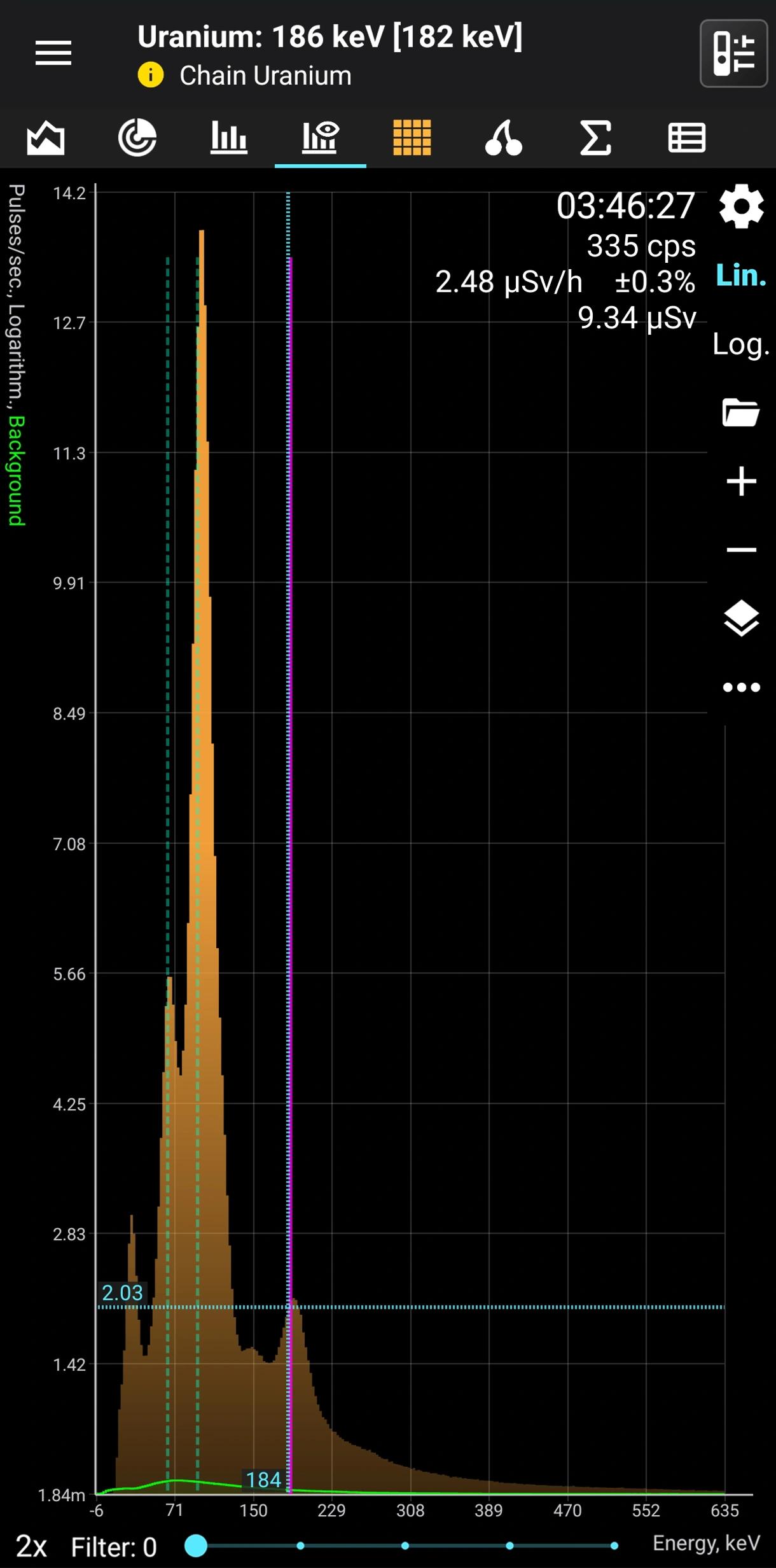
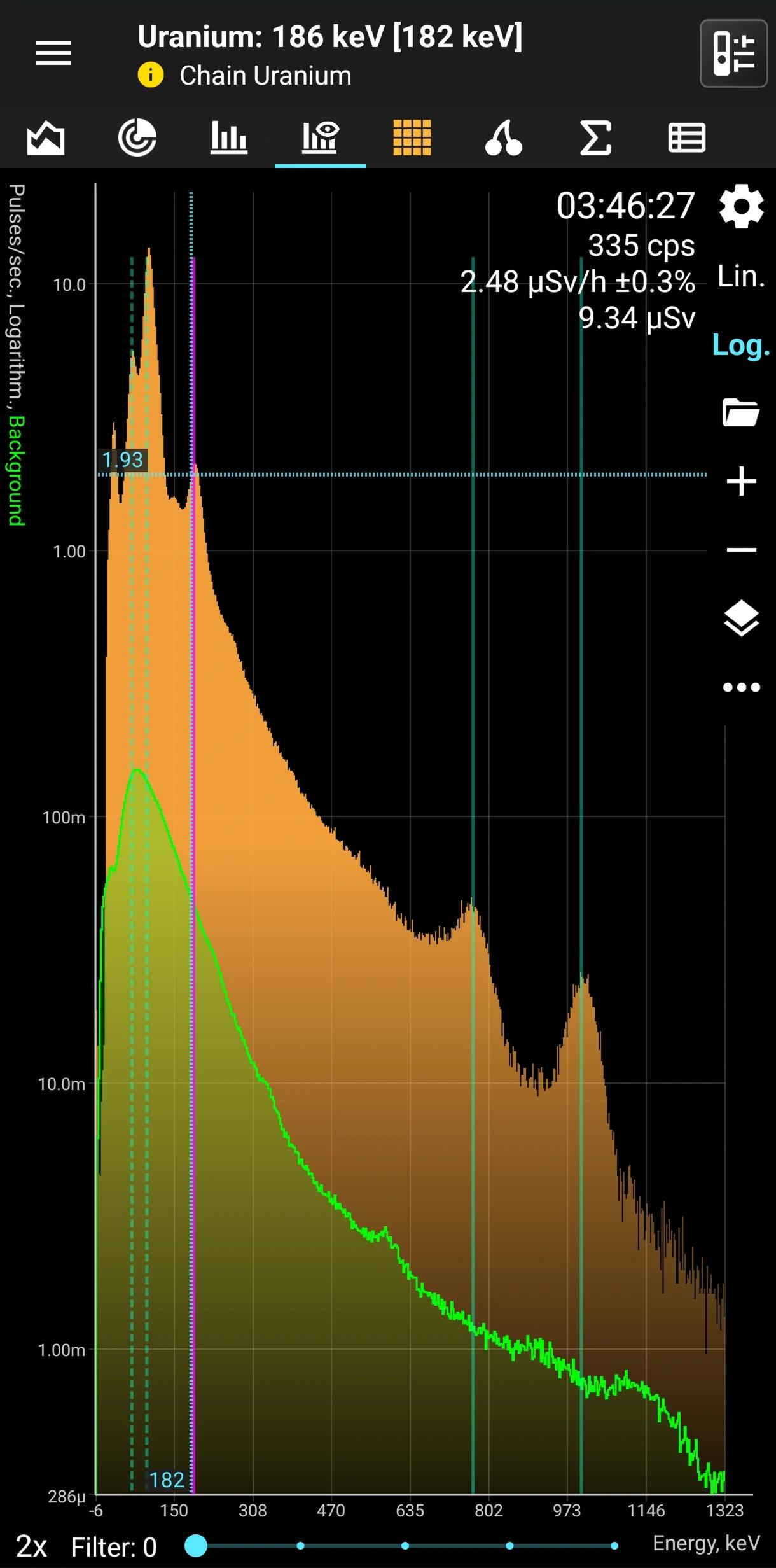
Uranium (without radium)
Natural α, β, γ radiation
Half-life: 4,5 billion years Main emission lines: 65, 95, 185, 750, 1001 keV
U-235. Decay mode Alpha
Alpha
| Energy, keV | Intensity, % |
| 4395.7 | 57.8 |
| 4364.6 | 18.94 |
| 4216.0 | 6.10 |
| 4597.7 | 4.77 |
| 4556.5 | 3.82 |
| 4323.2 | 3.58 |
| 4415.1 | 3.09 |
| 4502.9 | 1.28 |
Gamma
| Energy, keV | Intensity, % |
| 185.713 | 57.2 |
| 143.765 | 10.93 |
| 163.357 | 5.07 |
| 205.311 | 5.03 |
| 109.18 | 1.64 |
| 202.110 | 1.07 |
| 194.942 | 0.637 |
| 19.55 | 0.583 |
X-rays
| Energy, keV | Intensity, % |
| 11.118 - 20.450 | 27.7 |
| 93.347 | 4.8 |
| 89.954 | 3.0 |
| 104.817 - 108.971 | 2.30 |
| 104.817 - 106.315 | 1.72 |
| 108.479 - 108.680 | 0.58 |
U-238. Decay mode Alpha
Alpha
| Energy, keV | Intensity, % |
| 4198 | 79 |
| 4151 | 21 |
Gamma
| Energy, keV | Intensity, % |
| 49.55 | 0.064 |
| 113.5 | 0.0102 |
X-rays
| Energy, keV | Intensity, % |
| 11.118 - 20.450 | 7.3 |