
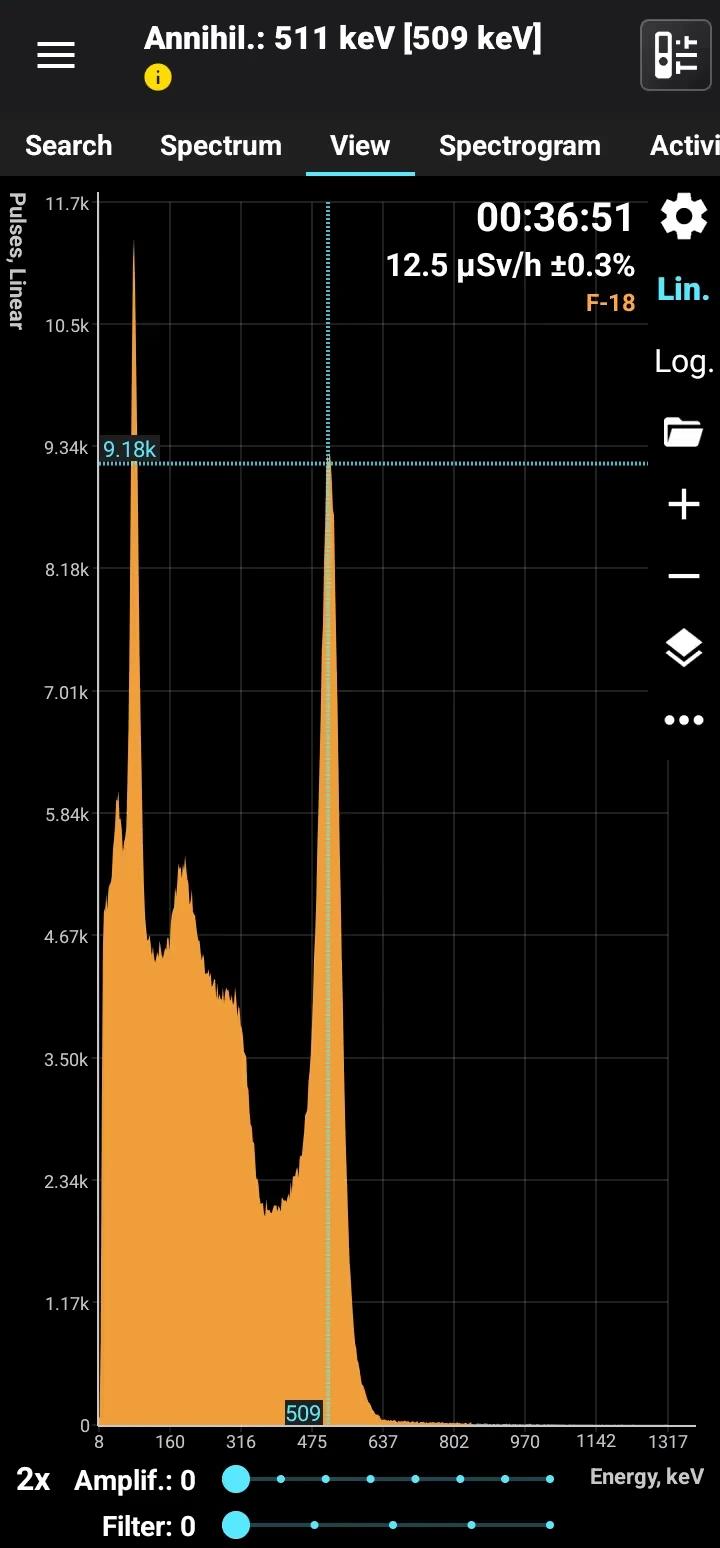
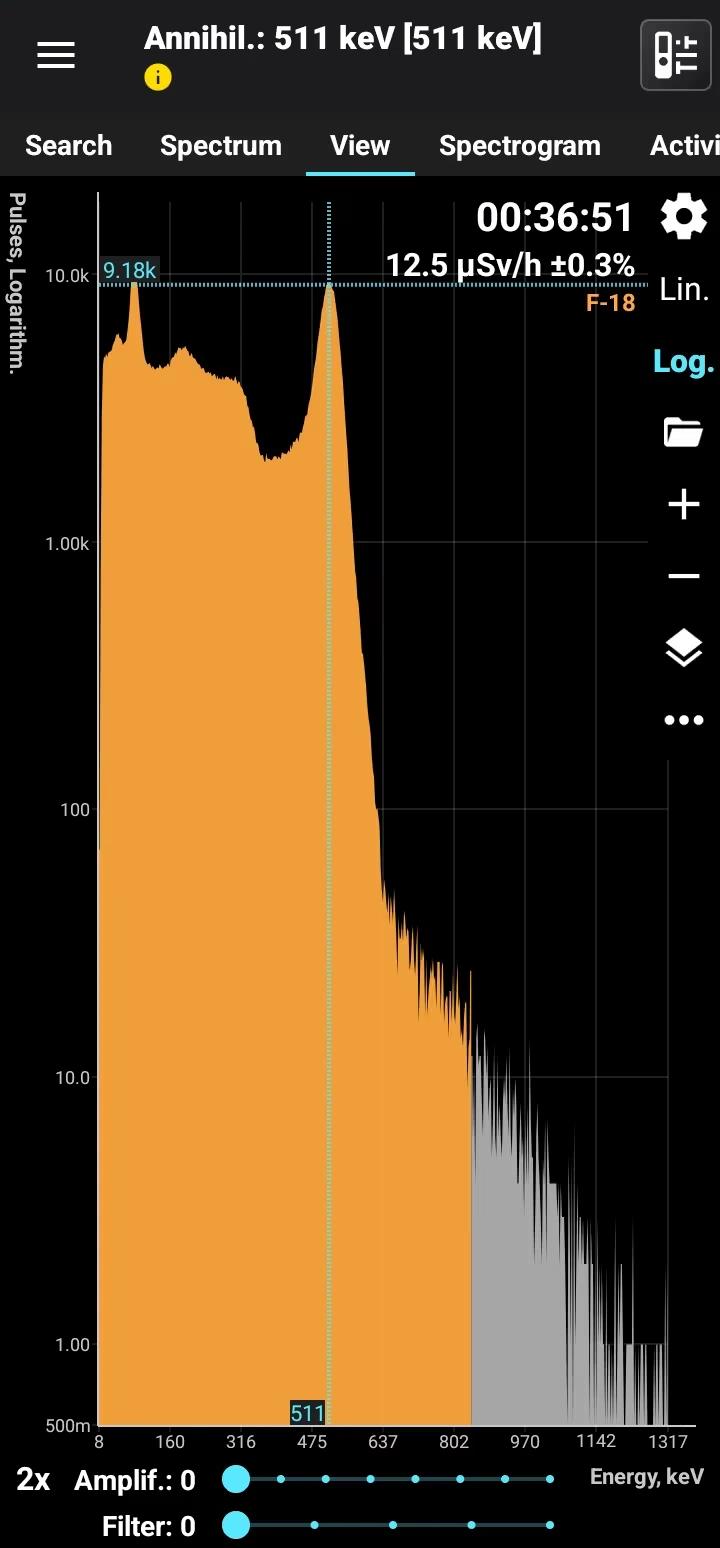
Fluorine-18 (F-18) is a radioactive isotope of fluorine with a half-life of approximately 109.8 minutes. It decays by positron emission (beta-plus decay) to stable oxygen-18 (O-18), emitting positrons and accompanying gamma radiation during annihilation. F-18 is produced artificially in cyclotrons by bombarding oxygen-18-enriched water with protons, resulting in its creation via nuclear reactions.

F-18 is widely used in nuclear medicine, particularly in positron emission tomography (PET) imaging. It is a key component in radiopharmaceuticals, such as F-18 fluorodeoxyglucose (FDG), which is commonly used to detect and monitor cancer, neurological disorders (e.g., Alzheimer's disease), and cardiovascular diseases. The high-energy positrons emitted by F-18 allow for high-resolution imaging, making it an essential tool in both clinical diagnostics and medical research. Beyond FDG, F-18 is used in other radiotracers for specialized imaging applications, such as studying brain function or mapping specific cellular receptors.

F-18 does not occur naturally due to its short half-life and is produced exclusively in controlled environments, such as cyclotron facilities. These facilities supply F-18 to hospitals and nuclear medicine departments for immediate use, as its short half-life necessitates rapid preparation and administration of radiopharmaceuticals. F-18 is encountered in medical imaging centers and research laboratories, where it is handled under strict safety regulations to ensure its safe use in clinical and research settings.
F-18
Fluorine-18
Half-life: 109 minutes Main emission lines: Annihilation 511 keV
Decay mode ec Beta+
Beta+
| Avg. En., keV | Intensity, % | Decay En., keV |
| 249.8 | 96.73 | (1655.4) |
Annihilation
| Energy., keV | Intensity, % |
| 511.0 | 193.46 |