
Lead-210 (Pb-210) is a radioactive isotope of lead with a half-life of approximately 22.3 years. It is part of the uranium-238 decay series, formed as a decay product of radon-222 through a series of intermediate isotopes. Pb-210 decays by beta emission to bismuth-210, which further decays in the chain. While it emits low-energy beta particles, its decay is accompanied by weak gamma radiation, making it detectable in certain contexts.

Pb-210 is primarily used in environmental and geological studies. Its relatively long half-life and specific decay properties make it valuable for dating sediments, such as lakebeds, wetlands, and ocean floors, to determine sedimentation rates over the past century. Pb-210 is also employed in studies of atmospheric deposition and the movement of heavy metals in the environment. Additionally, it is used in radiotracer studies to analyze natural processes and pollution pathways.

Pb-210 is naturally found as part of the uranium-238 decay chain and is present in trace amounts in soils, rocks, and sediments. It is also found in atmospheric aerosols due to the decay of radon-222, which escapes from the Earth's crust. Pb-210 can accumulate in biological systems and has been studied in relation to its presence in tobacco products, as it contributes to radiation exposure from smoking. It is commonly encountered in environmental monitoring, geological research, and areas with high uranium content in the soil or rock.
Pb-210
Plumbum-210
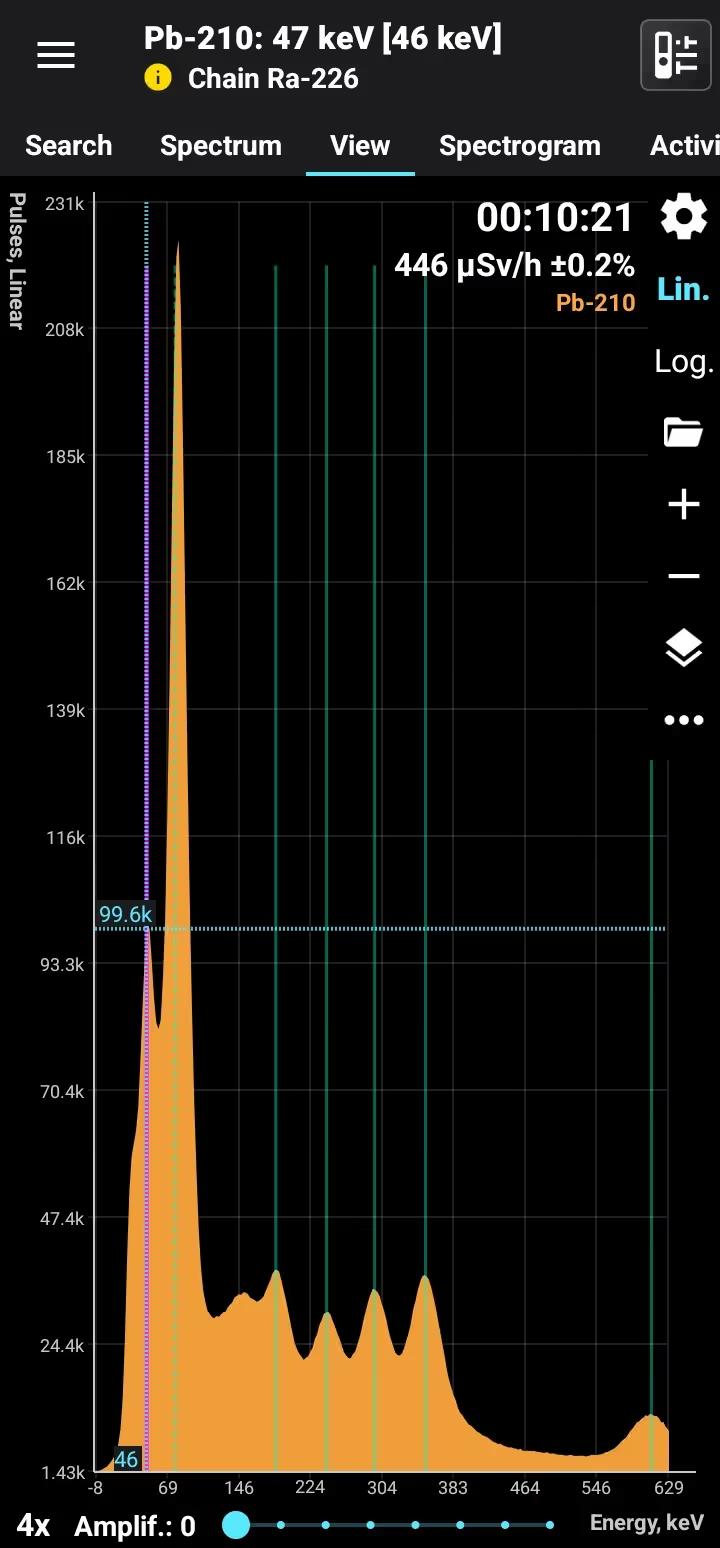
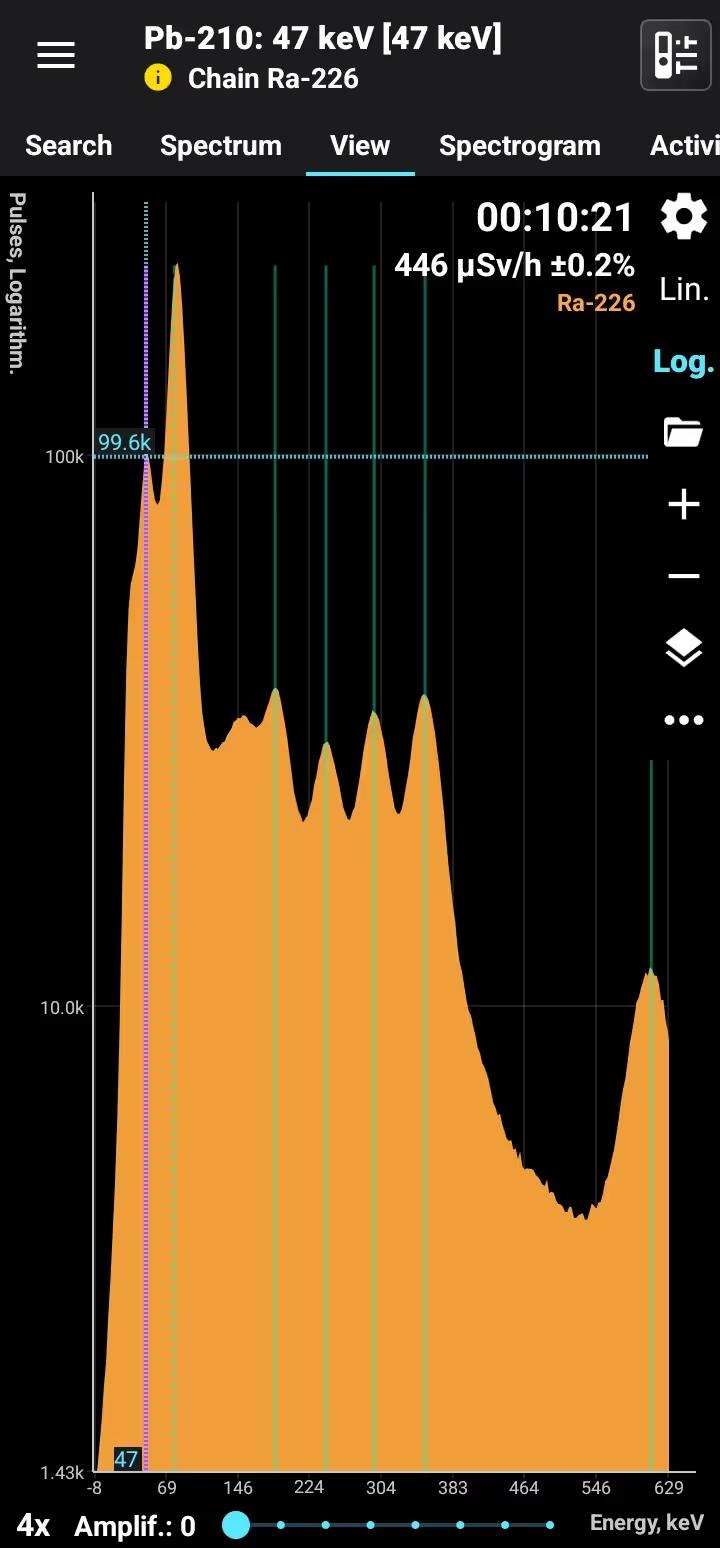
Half-life: 22.3 years Main emission lines: 47 keV Decay chain: Ra-226 Related lines: 78, 186, 242, 295, 351, 609, 1120, 1760, 2200 keV
Decay mode Alpha
Alpha
| Energy, keV | Intensity, % |
| 3720 | 0.0000019 |
Decay mode Beta-
Beta
| Avg. En., keV | Intensity, % | Decay En., keV |
| 4.16 | 84 | (17.0) |
| 16.16 | 16 | (63.5) |
Gamma
| Energy., keV | Intensity, % |
| 46.539 | 4.25 |
X-rays
| Energy., keV | Intensity, % |
| 9.419 - 16.389 | 22.7 |