
Selenium-75 (Se-75) is a radioactive isotope of selenium with a half-life of approximately 120 days. It decays by emitting gamma radiation, along with beta particles, as it transitions to stable arsenic-75 (As-75). The gamma emissions of Se-75, which occur at characteristic energies, make it suitable for various industrial and scientific applications. Se-75 is produced artificially in nuclear reactors through neutron activation of selenium-74.

Se-75 is widely used in industrial radiography for non-destructive testing (NDT). Its gamma rays provide a reliable method for inspecting the internal structure of materials, such as welds, pipes, and components, without causing physical damage. It is particularly valuable for imaging dense materials where other sources like X-rays may be less effective. Additionally, Se-75 is used in gamma spectrometry for calibration purposes and in scientific research to study selenium’s behavior in biological and chemical systems.

Se-75 does not occur naturally and is produced in controlled environments, such as nuclear reactors, by neutron activation of stable selenium isotopes. It is primarily encountered in industrial settings where it is used for radiographic inspections or in research laboratories for analytical studies. Its use is strictly regulated to ensure safety due to its radioactive nature and gamma radiation emissions.
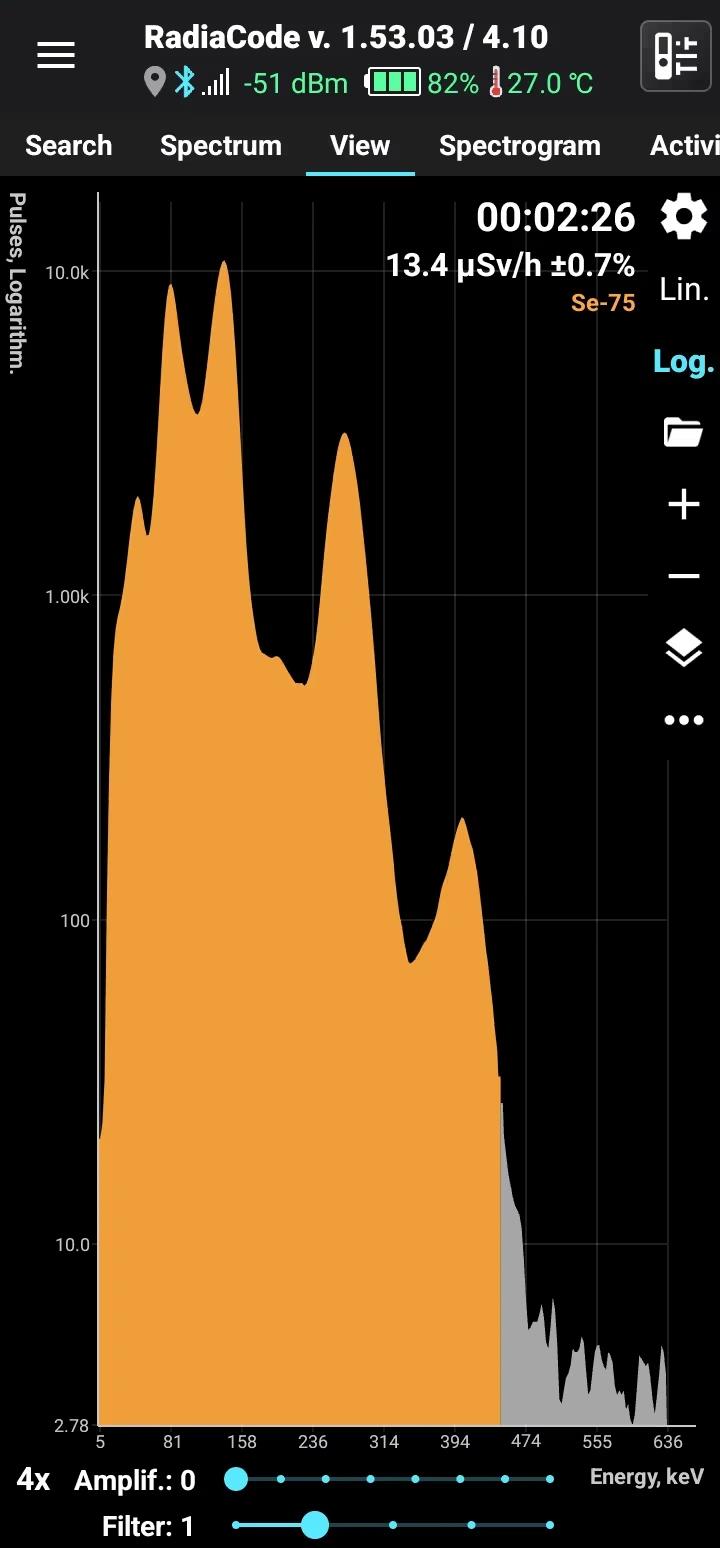
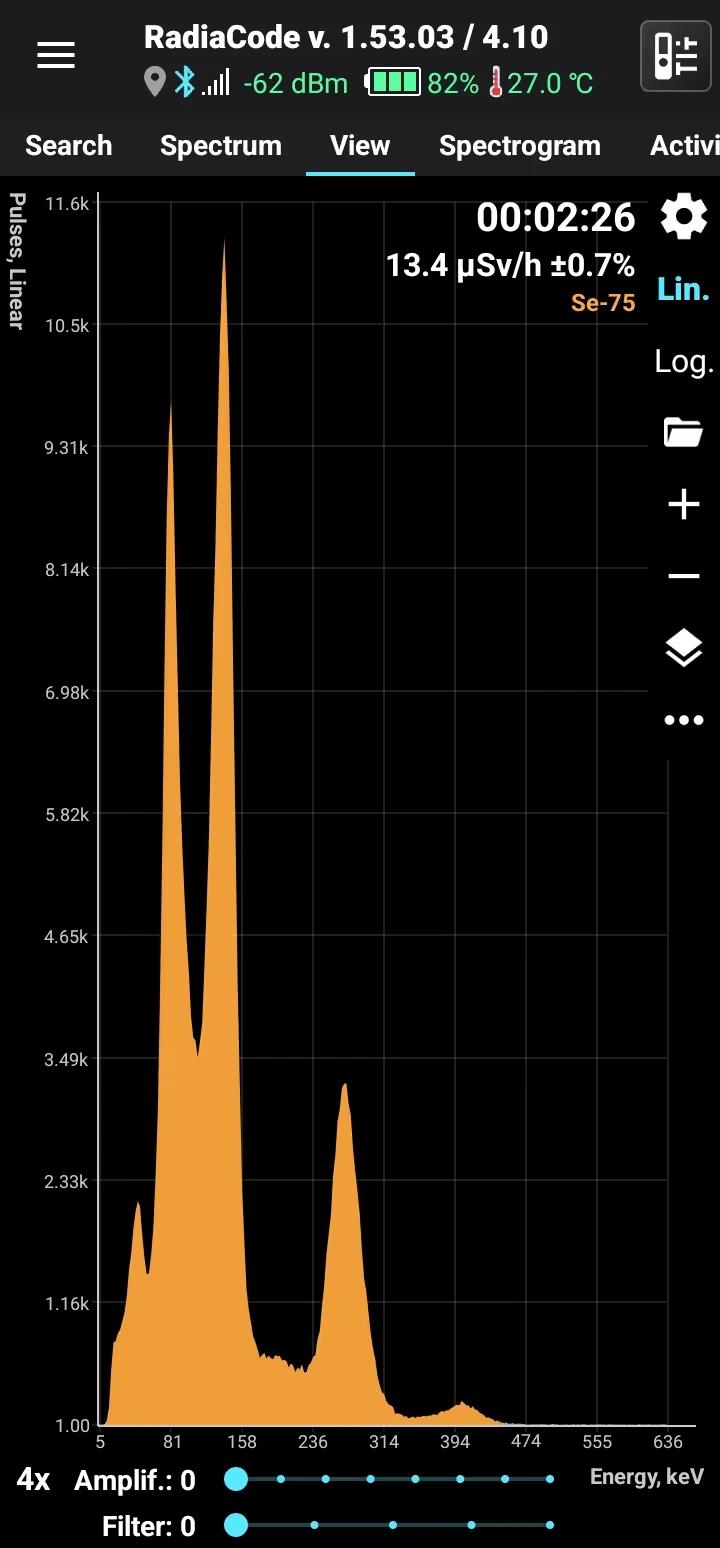
Se-75
Selenium-75
Half-life: 120 days Main emission lines: 136, 265, 401 keV
Decay mode ec
Gamma
| Energy., keV | Intensity, % |
| 264.6576 | 58.9 |
| 136.0001 | 58.5 |
| 279.5422 | 25.02 |
| 121.1155 | 17.20 |
| 400.6572 | 11.41 |
| 96.7340 | 3.45 |
| 198.6060 | 1.496 |
| 303.9236 | 1.315 |
| 66.0518 | 1.111 |
X-rays
| Energy., keV | Intensity, % |
| 10.544 | 32.1 |
| 10.509 | 16.53 |
| 11.720 - 11.861 | 7.61 |
| 11.720 - 11.826 | 7.33 |
| 1.119 - 1.521 | 2.05 |