
Silver-108m (Ag-108m) is a metastable radioactive isotope of silver with a half-life of approximately 418 years. The "m" denotes its metastable state, meaning the nucleus is in an excited energy state. It decays by emitting gamma radiation, which transitions the nucleus to its ground state, or by beta decay to cadmium-108 (Cd-108). The long half-life and gamma emission of Ag-108m make it useful in specific scientific and industrial applications.

Ag-108m is primarily used in scientific research, particularly in nuclear physics and radiation detection studies. Its gamma emissions make it suitable for use as a calibration source in gamma spectrometry. Additionally, it has potential applications in studying the behavior of silver isotopes in nuclear reactions and materials under radiation exposure. Ag-108m may also be of interest in experimental research on long-lived isotopes for potential energy storage or radiation shielding technologies.

Ag-108m is not naturally occurring and is produced artificially in nuclear reactors or particle accelerators through neutron activation of stable silver isotopes. It may be present in small quantities in nuclear waste or as a byproduct of certain nuclear reactions. Ag-108m is typically encountered in controlled environments, such as research laboratories or nuclear facilities, and its production and use are strictly regulated due to its long half-life and radiological properties.
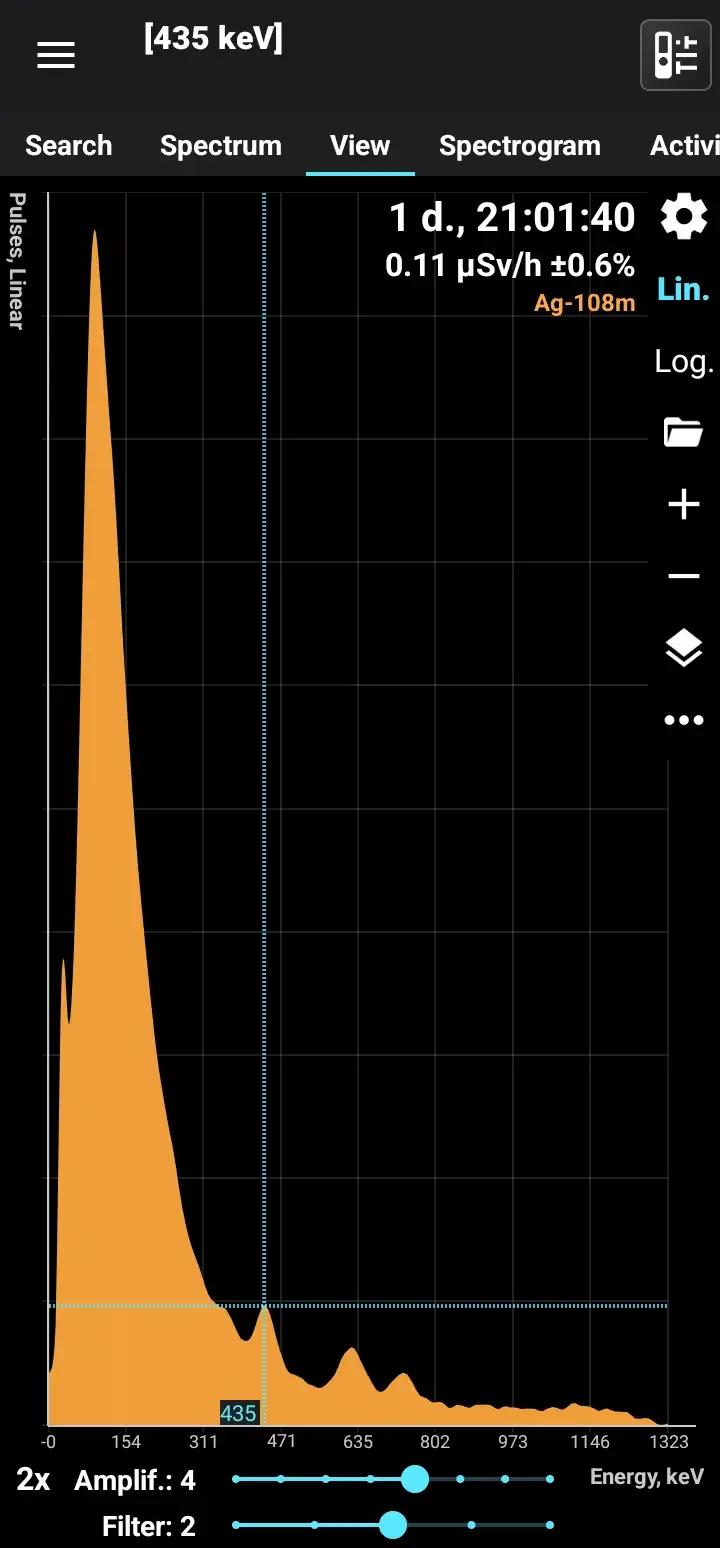
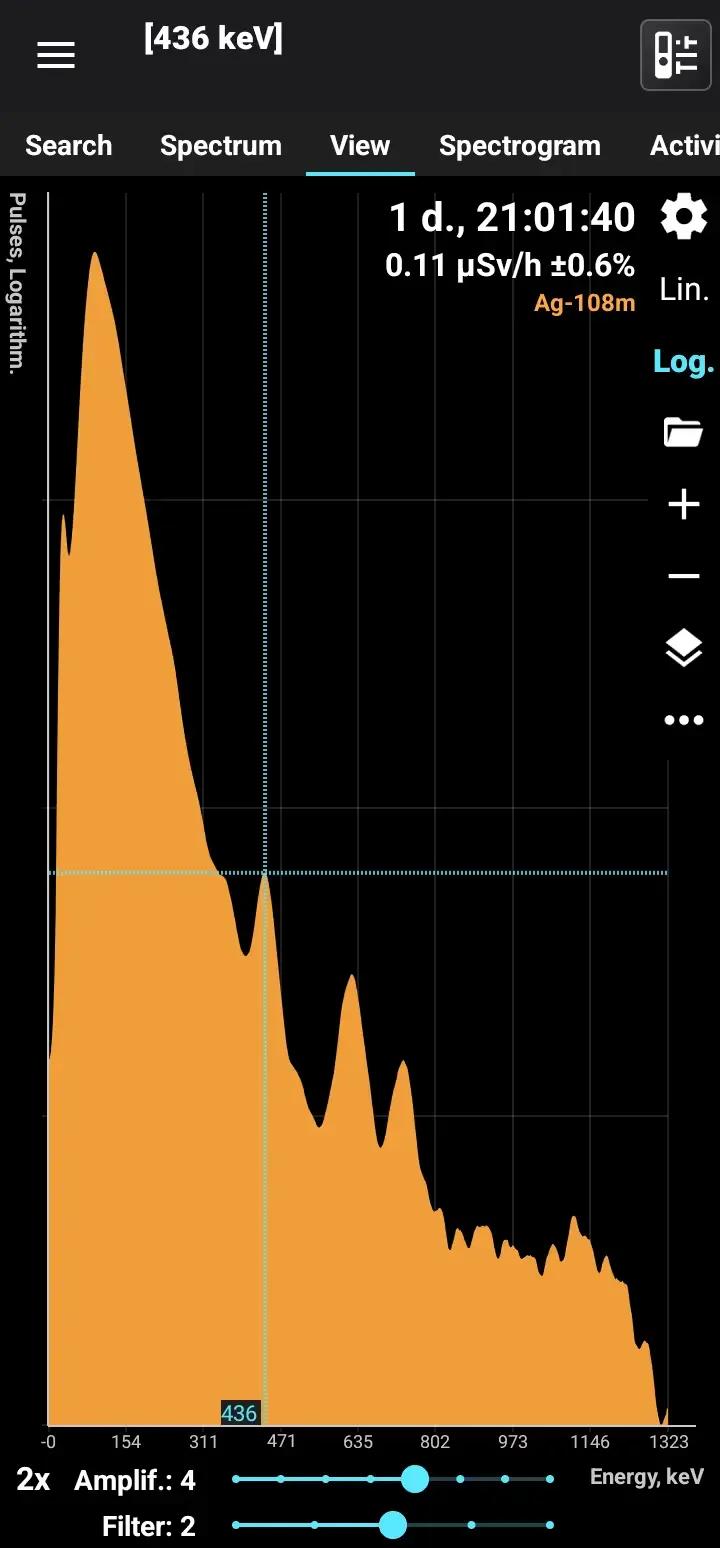
Ag-108m
Silver-108m
Half-life: 439 years Main emission lines: 80, 434, 614, 723 keV Decay chain: Ag-108 Related lines: 433, 618, 632 keV
Decay mode ec Beta+
Gamma
| Energy., keV | Intensity, % |
| 722.907 | 90.8 |
| 433.937 | 90.5 |
| 614.276 | 89.8 |
X-rays
| Energy., keV | Intensity, % |
| 21.177 | 34.7 |
| 21.020 | 18.39 |
| 23.792 - 24.348 | 11.15 |
| 23.792 - 24.015 | 9.53 |
| 2.503 - 3.602 | 4.71 |
| 24.294 - 24.300 | 1.62 |
Decay mode IT
Gamma
| Energy., keV | Intensity, % |
| 79.131 | 6.6 |
X-rays
| Energy., keV | Intensity, % |
| 22.163 | 0.91 |
| 2.633 - 3.802 | 0.51 |
| 21.990 | 0.48 |