
Gallium-68 (Ga-68) is a radioactive isotope of gallium with a half-life of approximately 68 minutes. It decays by positron emission (beta-plus decay) to stable zinc-68 (Zn-68), making it a valuable isotope for positron emission tomography (PET) imaging. Ga-68 is typically obtained from a germanium-68/gallium-68 generator, where it is produced as a decay product of germanium-68.
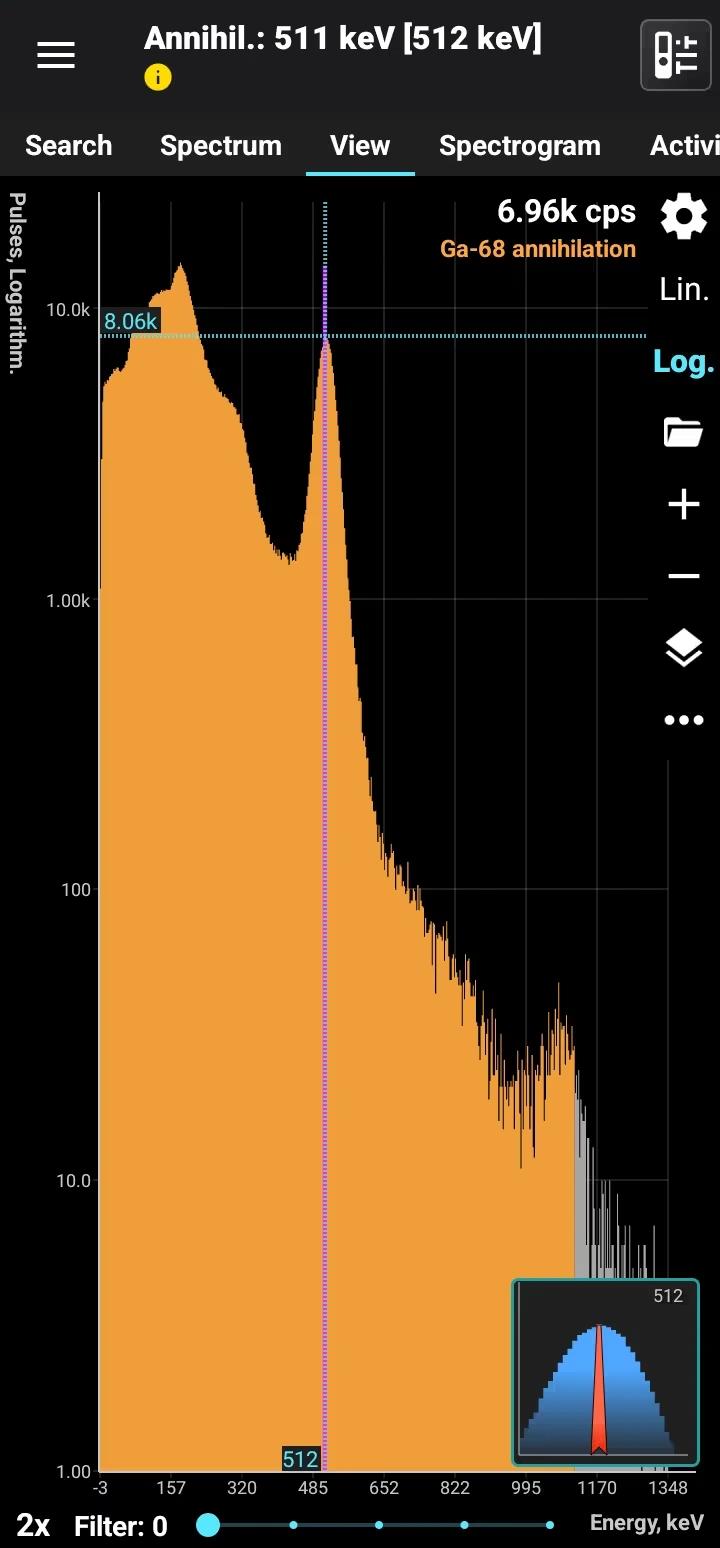
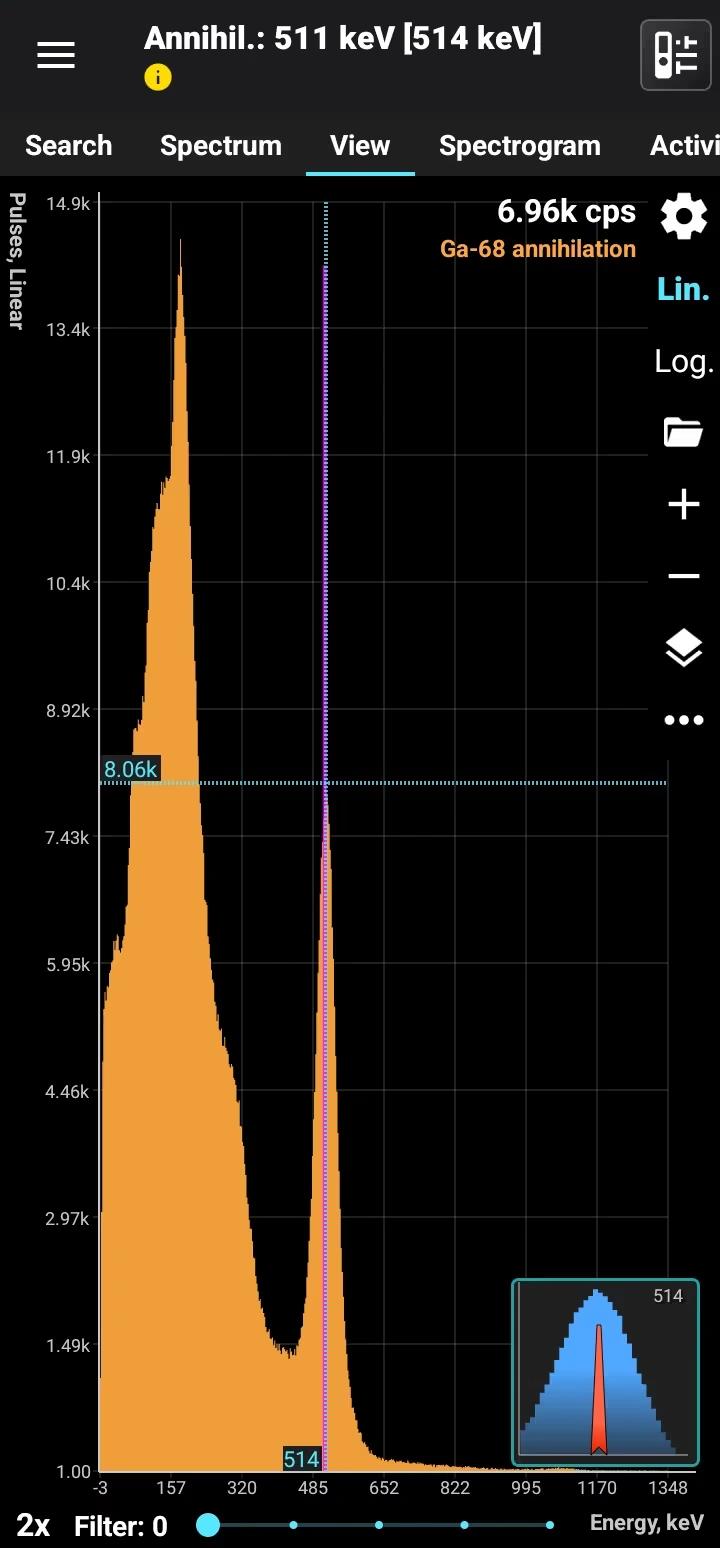
Gallium emits an annihilation line similar to Fluorine-18. These two isotopes can only be distinguished by evaluating their half-lives: Fluorine-18 has a half-life of 109 minutes, compared to 68 minutes for Gallium.

Ga-68 is widely used in nuclear medicine, particularly in PET imaging. It is a key isotope in radiopharmaceuticals for diagnosing cancer and other diseases. Ga-68-labeled compounds, such as Ga-68 DOTATATE and Ga-68 PSMA, are used for imaging neuroendocrine tumors and prostate cancer, respectively. These radiopharmaceuticals target specific receptors or antigens, enabling high-resolution imaging to detect and stage diseases with great accuracy. The short half-life of Ga-68 allows for rapid imaging while minimizing radiation exposure to the patient.

Ga-68 is not naturally occurring and is produced artificially. It is typically extracted from germanium-68/gallium-68 generators, which are used in hospitals and medical facilities. These generators provide a reliable on-site source of Ga-68 for radiopharmaceutical preparation. Due to its short half-life, Ga-68 is encountered only in controlled medical and research environments, and its production and use are strictly regulated to ensure safety and efficacy in clinical applications.
Ga-68
Gallium-68
Half-life: 68 minutes Main emission lines: 511 keV annihilation line
Decay mode ec Beta+
Beta+
| Avg. En., keV | Intensity, % | Decay En., keV |
| 836.0 | 87.72 | 2912 |
| 352.6 | 1.19 | (1843.7) |
Gamma
| Energy., keV | Intensity, % |
| 1077.34 | 3.22 |
| 1883.16 | 0.137 |
| 1261.08 | 0.0943 |
| 805.83 | 0.0940 |
| 578.52 | 0.0335 |
Annihilation
| Energy., keV | Intensity, % |
| 511.0 | 178 |
X-rays
| Energy., keV | Intensity, % |
| 8.639 | 2.757 |
| 8.616 | 1.418 |
| 9.569 - 9.652 | 0.592 |
| 0.883 - 1.187 | 0.152 |