
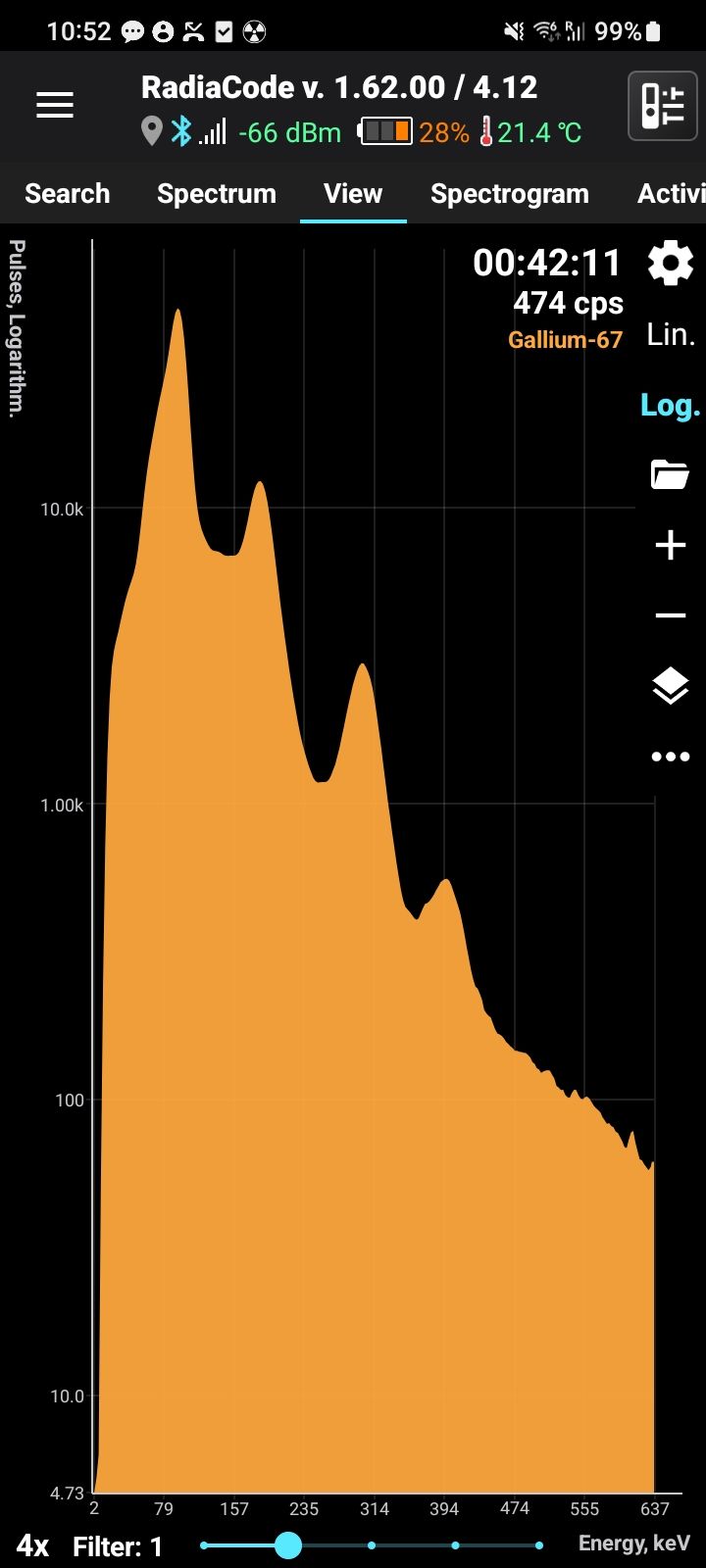
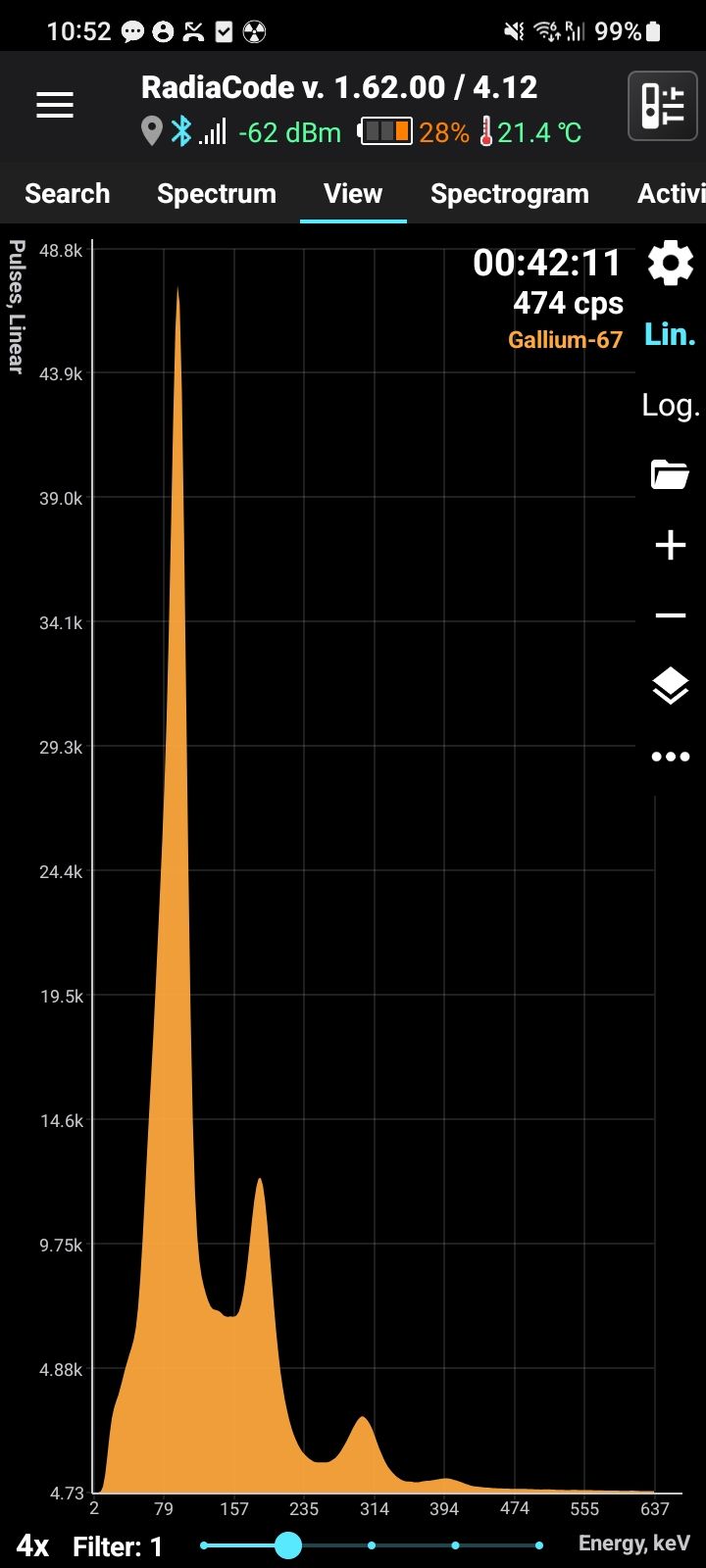
Gallium-67 (Ga-67) is a radioactive isotope of gallium with a half-life of approximately 78.3 hours (3.26 days). It decays by electron capture, emitting gamma radiation at characteristic energies, including 93 keV, 185 keV, 300 keV, and 394 keV. These gamma emissions make Ga-67 highly useful for medical imaging. Ga-67 is produced artificially in cyclotrons by proton irradiation of enriched zinc-68 (Zn-68).

Ga-67 is primarily used in nuclear medicine for tumor detection, infection imaging, and inflammatory disease diagnosis. It is a key component of Ga-67 citrate, a radiopharmaceutical injected into the body to localize various disease sites. Ga-67 imaging is particularly valuable in detecting Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, lung infections, and abscesses. It is also used in the evaluation of sarcoidosis and certain types of fever of unknown origin (FUO). Unlike Ga-68, which is used in PET imaging, Ga-67 is detected using single-photon emission computed tomography (SPECT).

Ga-67 does not occur naturally and is produced exclusively in cyclotrons through the proton bombardment of Zn-68. It is encountered in medical facilities, hospitals, and research laboratories specializing in nuclear medicine. Due to its longer half-life compared to Ga-68, Ga-67 can be transported and used in diagnostic imaging centers that do not have on-site production capabilities. Its use is strictly regulated to ensure safety in handling and patient administration.
Ga-67
Gallium-67
Half-life: 78.3 hours Main emission lines: 93, 185, 300, 394 keV
Decay mode ec
Gamma
| Energy, keV | Intensity, % |
| 93.310 | 38.81 |
| 184.576 | 21.41 |
| 300.217 | 16.64 |
| 393.527 | 4.56 |
| 91.265 | 3.11 |
| 208.950 | 2.46 |
X-rays
| Energy, keV | Intensity, % |
| 8.639 | 33.4 |
| 8.616 | 17.2 |
| 9.569 - 9.652 | 7.17 |
| 9.569 - 9.652 | 7.17 |
| 0.883 - 1.187 | 1.85 |